The post Powders: Manufacturing procedure and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>Molecular aggregation:
Precipitation and crystallization – Pharmaceutical powders can be produced by precipitation and crystallization. They are relatively similar processes, by making a solid solute precipitate out of solution.
Many approaches have been developed to accomplish precipitation or crystallization:
- Raising the concentration of solute in the solvent to a higher level, by removing a certain amount of solvent, such as by solvent evaporation.
- In most cases, the solubility of solid powder decreases, when the temperature of the solution is lowered, so, by cooling the solution, precipitation or crystallization can be achieved.
- Mixing the solution with another anti solvent in which the solid powder is insoluble or has very low solubility. For instance, for a poorly water-soluble drug, precipitation or crystallization can be obtained by adding water to the drug organic solution. In fact, there is a relatively new technology, called supercritical fluid method (SCF), based on this mechanism. Briefly speaking, a supercritical fluid is a substance maintained at a certain temperature and pressure above its critical point, which has both the properties of liquid and gas phases. It can penetrate like a gas and dissolve solids like a liquid. Supercritical fluid is a substitute for organic solvents to dissolve poorly water soluble drugs for powder generation, because the solubility of that powder in SCF can be easily altered by changing the temperature and pressure.
Spray drying – Spray drying is a technique to generate powders by transforming the feed from a liquid state into a dry form, by spraying the feed into a hot drying medium. The feed can be a solution, suspension, dispersion, or emulsion. The spray drying process mainly consists of five steps:
- Concentration—Before introduced to the spray dryer, feedstock is usually concentrated.
- Atomization—Favors evaporation to a dry powder, by having optimum properties.
- Droplet-Air Contact—In the chamber, atomized liquid contacts with hot gas, leading to evaporation of a majority of the water or solvent contained in the droplets within a few seconds.
- Droplet Drying—Water or solvent evaporation takes places.
- Separation—Cyclones, bag filters, and electrostatic precipitators may be used for the final separation stage.
Spray drying technology has many applications in the pharmaceutical industry, especially for powder technology. Spherical or non-spherical particles, hollow or solid particles, the size of the particles (frequently ranging 10–600 μm), and the uniformity of a powder can be altered by changing the parameters of the spray drier. By spray drying a poorly water-soluble drug, a coprecipitate with a polymer in a stable amorphous solid dispersion can be made. Therefore, the dissolution rate and bioavailability of many drugs can be greatly improved, such as tolbutamide, indomethacin, and ibuprofen. In addition, particles produced by spray drying can be controlled to have very a good aerodynamic performance, making them suitable for inhalation.
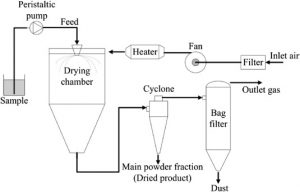
Fig 1 – Schematic diagram of spray drying (taken from science direct.com)
Spray freeze drying (SFD) – Spray freeze drying (SFD) is a technique based on the principle that the rapid solidification process induced by freezing prevents the molecules from packing in a certain order (refers to crystallization). SFD combined with freeze-drying, consists of the following steps:
- Atomization of feed using ultrasound, droplets are generated by one or two fluid nozzles or vibrating orifice (feed can be solution, suspension, or emulsion).
- Freezing of the droplets in a cryogenic liquid or cryogenic vapor, usually liquid nitrogen.
- Sublimation of solvent at low temperature and pressure by lyophilization or atmospheric freeze-drying using a cold desiccant gas stream.
- SFD has similar pharmaceutical applications as spray drying.
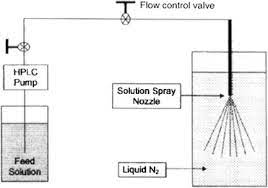
Fig 2 – Diagram of spray- freezing into liquid (taken from science direct.com)
Multiple choice questions;
1.Pharmaceutical powders can be produced by
a)precipitation
b)crystallization
c)fusion
d)a and b
2.Which of the following approaches have been developed to accomplish precipitation or crystallization?
a)Raising the concentration of solute in the solvent to a higher level, by removing a certain amount of solvent, such as by solvent evaporation.
b)In most cases, the solubility of solid powder decreases, when the temperature of the solution is lowered, so, by cooling the solution, precipitation or crystallization can be achieved.
c)Mixing the solution with another antisolvent in which the solid powder is insoluble or has very low solubility.
d)all of these
3.In most cases, the solubility of solid powder decreases, when the temperature of the solution is lowered, so, by cooling the solution, precipitation or crystallization can be achieved.
a)true
b)false
4.For a poorly water-soluble drug, precipitation or crystallization can be obtained by adding water to the drug organic solution. Which of the following new technology is based on this mechanism?
a)supercritical fluid method
b)supersaturated fluid method
c)both of these
d)none of these
5.A substance maintained at a certain temperature and pressure above its critical point, which has both the properties of liquid and gas phases is known as
a)supercritical fluid
b)supersaturated fluid
c)critical fluid
d)glassy state
6.Supercritical fluid is a substitute for organic solvents to dissolve poorly water soluble drugs for powder generation, because the solubility of that powder in SCF can be easily altered by changing the ____ and _____.
a)volume, pressure
b)temperature, pressure
c)pressure, density
d)volume, density
7.A technique to generate powders by transforming the feed from a liquid state into a dry form, by spraying the feed into a hot drying medium is called
a)Spray drying
b)Spray freeze drying
c)both of these
d)none of these
8.The feed in Spray drying can be a
a)solution
b)suspension
c)dispersion
d)all of these
9.The spray drying process mainly consists of ____ steps.
a)3
b)5
c)6
d)only one step
10.Which of the following is 1st step of spray drying?
a)Concentration
b)Atomization
c)Droplet-Air Contact
d)Droplet Drying
e)Separation
11.Which of the following steps in spray drying favors evaporation to a dry powder, by having optimum properties?
a)Concentration
b)Atomization
c)Droplet-Air Contact
d)Droplet Drying
e)Separation
12.Which of the following may be used for the final separation stage in spray drying?
a)Cyclones
b)bag filters
c)electrostatic precipitators
d)all of these
13.A technique based on the principle that the rapid solidification process induced by freezing prevents the molecules from packing in a certain order is called as
a)Spray drying
b)Spray freeze drying
c)both of these
d)none of these
14.Freezing of the droplets is done in a cryogenic liquid or cryogenic vapor, usually
a)argon
b)liquid nitrogen
c)helium
d)all of these
15.Spray freeze drying includes
a)sublimation
b)lyophilization
c)both of these
d)only a
Solutions:
- d)a and b
- d)all of these
- a)true
- a)supercritical fluid method
- a)supercritical fluid
- b)temperature, pressure
- a)Spray drying
- d)all of these
- b)5
- a)Concentration
- b)Atomization
- d)all of these
- b)Spray freeze drying
- b)liquid nitrogen
- c)both of these
References:
- Remington Essential of Pharmaceutics, 1st edition 2013, page no. 436-437.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
The post Powders: Manufacturing procedure and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>The post Powders: Advantages and limitations as dosage form, powders as dosage form and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>Advantages of powders:
- Unlike a standardized capsule or tablet powders enable a primary care provider to easily alter the quantity of medication for each dose.
- Powders can also aid in clinical studies of drug preparations because the dose can be so readily adjusted.
- Doses can be individually weighed and placed in powder papers, envelopes, or small vials/bottles.
- Infants and young children who cannot swallow tablets or capsules will accept powders that can be mixed with a formula or sprinkled in applesauce or some other appropriate food.
- If a drug is too bulky to be prepared as a capsule or tablet, it may be suitable for a powder dosage form.
- Powders provide a rapid onset of action because they are readily dispersed, have a large surface area, and usually require only dissolution, not disintegration, before.
Limitations of powders:
- Powders are not the dosage form of choice for drugs with unpleasant taste. This is because masking of unpleasant tastes may be a problem with this type of preparation.
- Drugs that deteriorate rapidly with exposure to atmosphere or acidic pH should not be dispensed as powders. For example, ferrous iron salts are easily oxidized and should not be administered as powders.
- Powders are bulky and inconvenient to carry.
- Powders are not a suitable dosage form for the administration of drugs that are inactivated in the stomach or drugs which can cause damage to the stomach.
- Dispensing potent drugs requiring low doses as powders (e.g., bulk powders) may not be appropriate. This is because individual doses are usually extracted from the bulk using a 5 ml spoon, which is subject to variation in spoon fill (e.g., level or heaped spoonfuls).
- Powders are not well suited for dispensing hygroscopic or deliquescent drugs.
Powders as dosage forms: There are a variety of powdered dosage forms commercially available, such as bulk powders, divided powders, dusting powders, insufflations, and
dry powder inhalers.
Bulk Powders – Bulk powders refer to a mixture of all the materials, packed into a properly designed bulk container, such as a glass or plastic bottle. The major problem of bulk powders is the inaccuracy of dose. Drugs present in the bulk powders are better suited, if they have a wider therapeutic window, a large dose, and pleasant taste. Effervescent powders are a special type of bulk powder. In addition to drugs and other excipients, effervescent powders contain an effervescent couple (i.e., sodium bicarbonate and citric acid), which react and effervesce when in contact with water. The effervescent dosage form is helpful to cover the unpleasant taste of salty or bitter drugs. For drugs that are not stable when dissolved in an aqueous pharmaceutically acceptable diluent, such as water, sterile liquid can be added to sterile powders contained in ampoules to form the solution just prior to use.
Divided Powders – Divided powders are bulk powders in which the individual dose has been packed separately. The traditional packing of divided powders is in wrapped paper. However, many problems are involved in this, when the materials are volatile, hygroscopic, or deliquescent. Therefore modern packing methods are developed to replace the use of paper wrapping, such as foil and plastic laminates. Effervescent powders can be packed into individual doses, because the plastic laminates can protect powders from moisture adsorption. The powdered product should always be protected from exposure to moisture.
Dusting Powders – Dusting powders are designed for external use, acting as a therapeutic, lubricant, or protective. Dusting powders act locally and are intended to have no systemic absorption. Dusting powders are usually dispensed in a relatively fine state (micronized) to increase efficacy and decrease irritation. Dusting powders can be packed in glass or metal containers with a perforated lid to allow the powders to be dusted to the effective area. Excellent flowability is necessary for this dosage form. Pressure aerosols are another delivery form that can generate dusting powders. They are more expensive than the sifter-container, but several advantages are realized, such as convenient operation, and protection from moisture, air, and contamination.
Insufflations and Dry Powder Inhalers (DPI) – Insufflations are fine powders of drugs, which are dosed into the nose, ear, or throat by the use of an insufflator. The use of conventional insufflators has declined, due to poor patient compliance and dose non-uniformity. Some newly developed devices have been introduced to replace the traditional insufflators. In these devices, drugs are usually dispensed with a carrier excipient, such as lactose, and placed into a hard gelatin capsule. When the device is operated, the capsule is broken and the fine powder is inhaled into the patient’s body.
Pulmonary delivery of dry powder formulations is a popular approach to deliver the drug to the lung locally, for the treatment of such diseases as asthma and chronic obstructive pulmonary disease (COPD).
Multiple choice questions:
1.A dry substance composed of finely divided particles is known as
a)tablet
b)capsule
c)powders
d)all of these
2.A medicated powder intended for internal use is
a)oral powder
b)topical powder
c)both of these
d)none of these
3.A medicated powder intended for external use is
a)oral powder
b)topical powder
c)both of these
d)none of these
4.Which of the following are advantages of powders?
a)Doses can be individually weighed and placed in powder papers, envelopes, or small vials/bottles
b)Powders are bulky and inconvenient to carry
c)Powders are not well suited for dispensing hygroscopic or deliquescent drugs
d)all of these
5.Infants and young children who cannot swallow tablets or capsules will accept ____ that can be mixed with a formula or sprinkled in applesauce or some other appropriate food.
a)pills
b)lozenges
c)powders
d)all of these
6.If a drug is too bulky to be prepared as a capsule or tablet, it may be suitable for a _____ dosage form.
a)parenteral
b)opthalmic
c)powders
d)suspensions
7.Powders provide a rapid onset of action because they
a)are readily dispersed
b)have a large surface area
c)usually require only dissolution not disintegration
d)all of these
8.Powders are not the dosage form of choice for drugs with unpleasant taste. This is because masking of unpleasant tastes may be a problem with this type of preparation.
a)true
b)false
9.Powders are not a suitable dosage form for the administration of drugs that
a)deteriorate rapidly with exposure to atmosphere or acidic pH
b)are inactivated in the stomach
c)can cause damage to the stomach
d)all of these
10.Powders are not well suited for dispensing
a)hygroscopic
b)deliquescent drugs
c)both of these
d)none of these
11.A mixture of all the materials, packed into a properly designed bulk container, such as a glass or plastic bottle is known as
a)Bulk powders
b)Divided powders
c)Dusting powders
d)Insufflations
12.Drugs present in the bulk powders are better suited, if they have
a)wider therapeutic window
b)a large dose
c)pleasant taste
d)all of these
13.Bulk powders in which the individual dose has been packed separately is known as
a)Bulk powders
b)Divided powders
c)Dusting powders
d)Insufflations
14.Powders that are designed for external use, acting as a therapeutic, lubricant, or protective are known as
a)Bulk powders
b)Divided powders
c)Dusting powders
d)Insufflations
15.Insufflations are fine powders of drugs, which are dosed into
a)nose
b)ear
c)throat
d)all of these
Solutions:
- c)powders
- a)oral powder
- b)topical powder
- a)Doses can be individually weighed and placed in powder papers, envelopes, or small vials/bottles
- c)powders
- c)powders
- d)all of these
- a)true
- d)all of these
- c)both of these
- a)Bulk powders
- d)all of these
- b)Divided powders
- c)Dusting powders
- d)all of these
References:
- Remington Essential of Pharmaceutics, 1st edition 2013, page no. 443.
- Ansels Pharmaceutical Dosage Forms and Drug Delivery systems, 10th edition, page no. 214-215.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
The post Powders: Advantages and limitations as dosage form, powders as dosage form and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>