The post General formulation of Emulsions and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>Oil Phase – Various chemical types of oils are used in the preparation of pharmaceutical emulsions, including hydrocarbons, simple esters, fatty acids, fixed and volatile oils, and waxes. The selection of oil phase is based on the solubility of the drug in the oil phase, oil/water partition coefficient of the drug, its tactile characteristics and feel, if the emulsion is meant for topical application. The most widely used oils in oral preparations are cod liver oils or various fixed oils of vegetable origin(e.g. cottonseed, arachis and maize oils) as nutritional supplements and non biodegradable mineral and castor oils that provide a local laxative effect. For topically applied emulsions, hydrocarbons such as hard and soft paraffin are widely used both as the vehicle for the drug and for their occlusive and sensory characteristics. Glycols are used to formulate non aqueous emulsions. The choice of oil is severely limited in parenteral emulsions. Purified soybean, sunflower, sesame and cottonseed oils composed mainly of long-chain triglycerides have been used for many years as they are resistant to rancidity and have few clinical side effects.
Table 1 – Ingredients for oil phase of emulsions
| Class | Identity | Consistency |
| Hydrocarbon | Mineral oils | Fluids of varying viscosities |
| Hydrocarbon | Petrolatum | Semisolid |
| Hydrocarbon | Polyethylene waxes | Solids |
| Hydrocarbon | Microcrystalline waxes | Solids |
| Ester | Vegetable oils | Fluids of varying viscosities |
| Ester | Animal fats | Fluids or solids |
| Ester | Lanolin | Semisolid |
| Ester | Synthetics (e.g. i-propylmyristate) | Fluids |
| Alcohols | Long chain (natural and synthetic) | Fluids or solids |
| Fatty acids | Long chain (natural and synthetic) | Fluids or solids |
| Ethers | Polyoxypropylenes | Fluids of varying viscosities |
| Silicones | Substituted | Fluids of varying viscosities |
| Mixed | Plant waxes (e.g. Candelilla) | Solid |
| Mixed | Animal waxes (e.g. bees) | Solid |
Emulsifiers – Emulsifiers are used both to promote emulsification at the time of manufacture and to control stability during a shelf life that can vary from days to months or years. For convenience, most pharmacy texts classify emulsifiers into three groups: (1) surface-active agents, (2) hydrophilic colloids (macromolecules) and (3) finely divided solids. The surfactants are primarily used as emulsifiers, whereas hydrophilic colloids and finely divided solids find their greatest utility in the form of auxiliary emulsifiers. The choice of emulsifier (surfactants) is determined by the type of emulsion desired, the required shelf life stability, the surfactant cost, the clinical use and the toxicity. For example, the addition of anionic surfactant is restricted to formulations meant for external use.
- The appropriate emulsifier or emulsifier mixture can be chosen by preparing a series of emulsions with a range of surfactants of varying HLBs.
- Mixtures of surfactants with high HLB and low HLB give more stable emulsions than do single surfactants.
- The solubility of surfactant components in both the disperse phase and the continuous phase maintains the stability of the surfactant film at the interface.
- The formation of a viscous network of surfactants in the continuous phase prevents their collision, and this effect overrides the influence of the interfacial layer and barrier forces due to the presence of adsorbed layers.
Four categories of surface-active agents are used to stabilize pharmaceutical emulsion/cream formulations: (1) anionic, (2) cationic, (3) nonionic and (4) amphoteric.
Determination of emulsifier amount – The least amount of surfactant mixture required for optimal stability of an emulsion is determined by the amount of water that can be solubilized in a given oil-plus-surfactant(s) mixture under carefully controlled temperature and stirring conditions. For this purpose, 10 g of the oil–surfactant mixture is weighed in a glass vial. Water is added in 0.1 mL increments. The mixture is shaken and allowed to stand at the equilibration temperature (temperature at which this system is fluid) until all air bubbles have escaped. The addition of water (0.1 mL increments) is continued until the system remains permanently turbid. If the initial oil–surfactant mixture is not clear, it will usually become clear upon the addition of water and then will become cloudy again upon continued addition of water. This second cloudpoint is the end of titration. As a rule, the most stable o/w emulsion with the finest particle size results at that oil–surfactant ratio that can tolerate the largest quantity of water and still remain clear.
Auxiliary Emulsifiers –
Hydrophilic colloids: Polymers that are water sensitive (swellable or soluble) have some utility as primary emulsifiers; however, their major use is as an auxiliary emulsifier and as a thickening agent. Clays such as bentonite swell in the presence of water and are used for building the viscosity of emulsions. Other clays such as attapulgite thicken primarily because of particle anisotropy.
Table 2 – Hydrophilic colloids useful in emulsion technology
| Class | Emulsiłer name |
| Polysaccharide | Gum arabic (acacia)
Gum karaya Gum tragacanth Guar gum Carrageenan |
| Protein | Gelatin |
| Cellulose | Methyl cellulose
Hydroxyethyl cellulose Hydroxypropyl cellulose Carboxymethyl cellulose |
| Synthetic | Polyoxethylene polymer
Carboxyvinyl polymer |
Finely divided solids – Finely divided solids have been shown to be good emulsifiers, especially in combination with surfactants and/or macromolecules that increase viscosity. This includes polar inorganic solids, such as heavy metal hydroxides, certain nonswelling clays and pigments. Even nonpolar solids (e.g. carbon or glyceryltristearate) can be used. Polar solids tend to be wetted by water to a greater extent than by the oil phase, whereas the reverse is true for nonpolar solids. In the absence of surfactants, w/o-type emulsions are favoured by the presence of nonpolar solids, presumably because the wetting by oil facilitates the coalescence of oil droplets during the initial steps of emulsification. An analogous interpretation may be given for the tendency of polar solids to favour water as the external phase.
Viscosity Modifiers – Once the desired emulsion and emulsifiers have been chosen, a consistency that provides the desired stability and yet has the appropriate flow characteristics must be attained. It is well known that the creaming of fluid emulsions depends on the surface characteristics of the interfacial film as well as on the rheological character. The creaming rate of suspended globules is inversely proportional to the viscosity in accordance with Stokes’ law. When all other variables are held constant, an increase in viscosity generally minimizes creaming, rising or sedimentation. In the case of o/w emulsions, gums and clays are added to increase viscosity, whereas for w/o emulsions, polyvalent metal soaps or high melting waxes and resins are used.
Preservatives – Emulsions often contain a number of ingredients, such as proteins, carbohydrates, phosphatides and sterols, all of which readily support the growth of various microbes. Even in the absence of any of the aforementioned natural ingredients, the intimate contact of an oil and water allows microbes to establish themselves. As a result, the inclusion of a preservative is a necessary part of the formulation process. The preservative must first meet the general criteria of low toxicity, chemical compatibility, stability to heat and storage, acceptable taste, odour and colour, and reasonable cost. Since microorganisms can reside in water, in the oil phase or in both, it is customary that the preservative should be available at an effective level in both phases. The esters of p-hydroxybenzoic acid are particularly good examples because methyl ester (methyl paraben) is water soluble, whereas propyl ester (propyl paraben) is oil soluble.
Antioxidant – Oils are subjected to autoxidation upon exposure to air. Upon autoxidation, unsaturated oils, such as vegetable oils, give rise to rancidity, resulting in unpleasant odour, appearance and taste. Autoxidation is a free radical chain oxidation reaction. It can be inhibited, therefore, by the absence of oxygen, by a free radical chain breaker or by a reducing agent. The choice of a particular antioxidant depends on its safety, acceptability for a particular use and its efficacy. Antioxidants are commonly used at concentrations ranging from 0.001 to 0.1%. Butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), L-tocopherol and alkyl gallates are particularly popular in pharmaceuticals and cosmetics. BHT and BHA have a pronounced odour and should be used at low concentrations. Alkyl gallates have a bitter taste, whereas L-tocopherol is well suited for oral emulsions.
Multiple Choice Questions:
1.Which of the following components are used in formulation of emulsions?
a)Oil Phase
b)Viscosity Modifiers
c)Finely divided solids
d)All of these
2.Various chemical types of oils are used in the preparation of pharmaceutical emulsions, including
a)hydrocarbons
b)simple esters
c)fatty acids
d)all of these
3.The selection of oil phase is based on the solubility of the drug in the oil phase, oil/water partition coefficient of the drug.
a)true
b)false
4.The most widely used oils in oral preparations are
a)cottonseed
b)arachis
c)maize oils
d)all of these
5.For topically applied emulsions which of the following oil phase is/are used?
a)hard paraffin
b)soft paraffin
c)both of these
d)none of these
6.Which of the following oils are resistant to rancidity?
a)soybean oil
b)sunflower oil
c)sesame oil
d)all of these
7.Which of the following is not an ester oil?
a)Vegetable oils
b)Animal fats
c)Lanolin
d)Polyoxypropylenes
8.Which of the following is plant wax?
a) Candelilla
b)Bees
c) i-propylmyristate
d)All of these
9.The surfactants are primarily used as emulsifiers, whereas hydrophilic colloids and finely divided solids find their greatest utility in the form of auxiliary emulsifiers.
a)true
b)false
10.The choice of emulsifier (surfactants) is determined by
a)the type of emulsion desired
b)the required shelf life stability
c)the surfactant cost
d)all of these
11.The least amount of surfactant mixture required for optimal stability of an emulsion is determined by the amount of water that can be solubilized in a given oil-plus-surfactant(s) mixture under carefully controlled temperature and stirring conditions.
a)true
b)false
12.Auxiliary Emulsifiers are
a)Hydrophilic colloids
b)Hydrophobic colloids
c)Both of these
d)None of these
13.Gum karaya is
a)Polysaccharide
b)Protein
c)Cellulose
d)Synthetic
14.Gelatin is
a)Polysaccharide
b)Protein
c)Cellulose
d)Synthetic
15._______is well suited for oral emulsions.
a)Alkyl gallates
b)L-tocopherol
c)BHA
d)All of these
Solutions:
- d)All of these
- d)all of these
- a)true
- d)all of these
- c)both of these
- d)all of these
- d)Polyoxypropylenes
- a) Candelilla
- a)true
- d)all of these
- a)true
- a)Hydrophilic colloids
- a)Polysaccharide
- b)Protein
- b)L-tocopherol
References:
- Gaurav K. Jain Theory and Practice of Physical Pharmacy, 1st edition 2012 Elsevier, page no. 229-233.
- Remington Essential of Pharmaceutics, 1st edition 2013, page no. 450, 451.
- Ansel’s Pharmaceutical Dosage Forms and Drug Delivery systems, 10th edition, page no. 468-470.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
The post General formulation of Emulsions and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>The post Preservatives, Types of Preservatives, Properties of Preservatives and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>Examples – Alcohol, Benzalkonium chloride, Boric acid, Butylated Hydroxyanisole (BHA), Butylated Hydroxytoluene (BHT), Butylparaben, Methylparaen, Phenol, Phenethyl Alcohol, Potassium Sorbate, Propylene Glycol, Propylparaben, Sorbic Acid.
1.Alcohol: INN: Alcohol; Ethanol; Ethanolum
Synonyms: Ethanol; Ethyl alcohol, grain alcohol
Chemical Name CAS Number: Ethanol
Molecular Formula: C2H6O
Description: A clear, volatile liquid with a characteristic odor and burning taste.
Properties: Miscible with water and glycerin; boiling point 78°C.
Incompatibilities: Acacia solutions (precipitation of the acacia gum), oxidizing materials while in acidic conditions.
Use: As a preservative in concentration greater than 10%; as a disinfectant in concentrations of 60–90%; as a co-solvent for certain active pharmaceutical ingredients or in film coating and tablet printing unit operations; and as a vehicle for both oral and topical finished products.
2.Benzalkonium chloride:
INN: Benzalkonium chloride; Benzalkonii chloridum
Synonyms: Alkylbenzyldimethylammonium chloride; BKC; BAC
Chemical Name & CAS Number: alkyldimethyl(phenylmethyl) ammonium chloride (8001-545 ammonium chloride (8001-54-5)
Molecular Formula: [C6H5CH2N(CH3)2R]Cl, where R can be–C8H17, C12H25, C14H29 and C16H23
Structure:
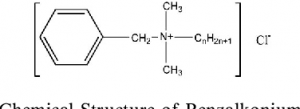
Description: A white to yellow-white powder, flake, or gel.
Properties: 10% solution pH 5–8; hygroscopic; soapy to touch; density 0.98g/cc; very soluble in water and alcohol.
Incompatibilities: Aluminum salts, anionic surfactants, citrates, iodides, hypromellose, hydrogen peroxide, lanolin, nitrates, and nonionic surfactants in high concentrations, proteins, permanganates, salicylates, and tartrates.
Use: Preservative.
3.Boric Acid:
INN: Boric acid; Acidum boricum
Synonyms: Boracic Acid; Orthoboric Acid; Boracic acid; boron trihydroxide
Chemical Name & CAS Number: Boric acid (10043-35-3)
Molecular Formula: H3BO3
Description: Hygroscopic white crystalline powder
Properties: Soluble 1 g in 18 mL water, 18 mL alcohol; 5% solution pH 3.5-4.1; density 1.435 g/cc.
Incompatibilities: Strong bases, alkali, reacts violently with potassium and acid anhydrides and complexes with glycerin.
Uses: As a buffer and preservative for eye drops.
4.Butylated Hydroxyanisole (BHA), Butylated Hydroxytoluene (BHT): Discussed in antioxidant article already. Please refer that article.
5.Butylparaben:
INN: Butyl Parahydroxybenzoate; butylparaben
Synonyms: Butoben, 4-hydroxybenzoic acid butyl ester
Chemical Name & CAS Number: Butyl-4-hydroxybenzoate(94-26-8)
Molecular Formula: C11H14O3
Structure:

Description: White to off-white crystalline powder that is odorless and tasteless.
Properties: Very slightly soluble in water or glycerin, freely soluble in propylene glycol, melting range 68–72°C.
Incompatibilities: Avoid nonionic surfactants, as these reduce antimicrobial effectiveness, due to micellization. Possible absorption of butylparaben by plastic bottles is under investigation.
Use: Antimicrobial preservative for liquid and semi-solid preparations.
6.Methylparaben:
INN: Methyl hydroxybenzoate; methyl parahydroxybenzoate; Methylis parahydroxybenzoas
Synonyms: 4-hydroxybenzoic acid methyl ester; methyl-p-hydroxybenzoate
Chemical Name & CAS Number: Methyl 4-hydroxybenzoate(99-76-3)
Molecular Formula: C8H8O3
Structure:
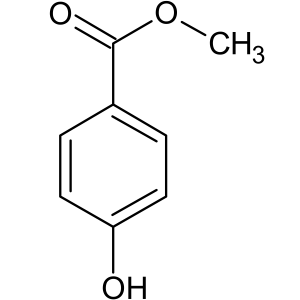
Description: White to off-white crystalline powder that has a slight burning taste.
Properties: Antimicrobial activity against most organisms, most effective in ph range of 4–8; density of 1.352 g/cc; pKa 8.4 at 22°C; solubility in water 1 in 400, in propylene glycol 1 in 5, in glycerin 1 in 60, in ethanol 1 in 2.
Incompatibilities: Nonionic surfactants (e.g., polysorbate), talc, bentonite, tragacanth gum, sodium alginate, sorbitol, and methylcellulose.
Uses: As an antimicrobial preservative in all known delivery systems.
7.Phenol:
INN: Phenol; Phenolum
Synonyms: Carbolic Acid; hydroxybenzene; oxybenezene; phenic acid; phenyl hydrate; phenyl hydroxide; phenylic acid; phenylic alcohol
Chemical Name & CAS Number: Phenol (108-95-2)
Molecular Formula: C6H6O
Structure:
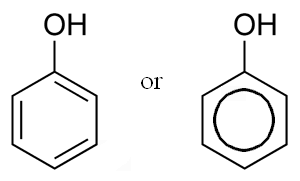
Description: Colorless to light pink, interlaced, or separate, needle-shaped crystals, or a white or light pink, crystalline mass; characteristic odor; when undiluted, it whitens and cauterizes the skin and mucous membranes; when gently heated, phenol melts, forming a highly refractive liquid; liquefied by the addition of 10% of water.
Properties: Solubility 1 g in 15 mL water; very soluble in alcohol, glycerin, chloroform, ether, or fixed and volatile oils; sparingly soluble in mineral oil; vapor is flammable; gradually darkens on exposure to light and air; specific gravity 1.07; boils at 182°C; congeals at not less than 39°C.
Incompatibilities: Produces a liquid or soft mass when triturated with camphor, menthol, acetanilide, acetophenetidin, aminopyrine, antipyrine, ethyl aminobenzoate, methenamine, phenyl salicylate, resorcinol, terpin hydrate, thymol, and several other substances, including some alkaloids. It also softens cocoa butter in suppository mixtures. Traces of iron in various chemicals (such as alum, borax, etc. may produce a pink to violet color.
Uses: As a caustic, disinfectant, topical anesthetic; a preservative for some injections; used in several proprietary antiseptic mouthwashes, hemorrhoidal preparations, and burn remedies.
8.Phenylethyl Alcohol:
INN: Phenylethyl alcohol
Synonyms: Benzeneethanol; phenethanol; benzyl carbinol; benzylmethanol; β-hydroxyethyl benzene; 2-phenylethyl alcohol; pheneylethanol; PEA
Chemical Name & CAS Number: Phenethyl alcohol [60-12-8]
Molecular Formula: C8H10O
Structure:
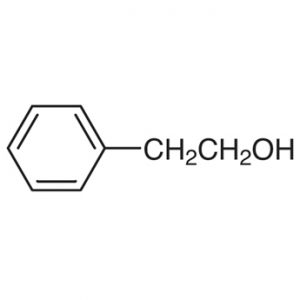
Description: Colorless liquid with a rose-like odor and a sharp, burning taste.
Properties: Solubility is 1 g in 60 mL water or 1 mL alcohol, chloroform, or ether; very soluble in fixed oils, glycerin, or propylene glycol; slightly soluble in mineral oil; solidifies at 27°C; specific gravity 1.017-1.020.
Uses: As an antimicrobial preservative.
9.Potassium sorbate:
INN: Potassium sorbate; Kalii sorbas
Synonyms: 2,4-hexadienoic acid (E,E)-potassium salt; potassium (E,E) sorbate
Chemical Name & CAS Number: Potassium (E,E)-hexa-2,4- dienoate (24634-61-5)
Molecular Formula: C6H7KO2
Structure:
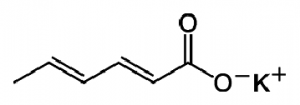
Description: White crystals or powder with a characteristic odor.
Properties: Solubility 1 g in 1.7 mL water, 1.8 mL of propylene glycol or 35 mL alcohol; density 1.36 g/cc; melting point ca. 270°C with decomposition.
Uses: As a preservative.
10.Propylene glycol:
INN: Propylene glycol; Propyleneglycolum
Synonyms: 1,2-Dihydroxypropane; 2-hydroxypropanol; methyl ethylene glycol; methyl glycol; propane-1,2-diol
Chemical Name & CAS Number: 1,2-Propanediol (57-55-6)
Molecular Formula: CH3CH(OH)CH2OH
Structure:
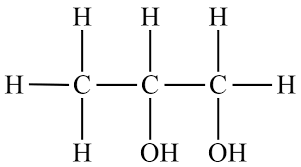
Description: Clear, colorless, viscous, and practically odorless hygroscopic liquid; with a slightly acrid taste.
Properties: Miscible with water, alcohol, acetone, or chloroform; soluble in ether; dissolves many volatile oils; immiscible with fixed oils; specific gravity 1.035-1.037; viscosity approximately 58 mPa s.
Uses: As a solvent; preservative; humectants.
11.Propylparaben:
INN: Propyl hydroxybenzoate; Propyl parahydroxybenzoate; Propylis parahydroxybenzoas; Propylparaben
Synonyms: 4-hydroxybenzoic acid propyl ester; propagin; propyl ρ-hydroxybenzoate
Chemical Name & CAS Number: Propyl 4-hydroxybenzoate(94-13-3)
Molecular Formula: C10H12O3
Structure:
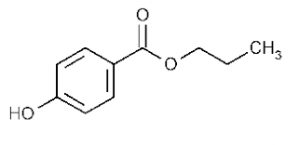
Description: White crystalline odorless and tasteless powder
Properties: Very poorly soluble in water, 1 in 250 of glycerin, 1 in 4 of propylene glycol; density 1.288 g/cc.
Incompatibilities: Nonionic surfactants (incorporation into micelles); may be absorbed by some plastics and discolors in the presence of iron.
Use: As an antimicrobial agent.
12.Sorbic Acid:
INN: Sorbic acid; Acidum sorbicum
Synonyms: 2,4-hexadienoic acid; 2-propenylacrylic acid
Chemical Name & CAS Number: (E,E)-Hexa-2,4-dienoic acid(22500-92-1)
Molecular Formula: C6H8O2
Structure:
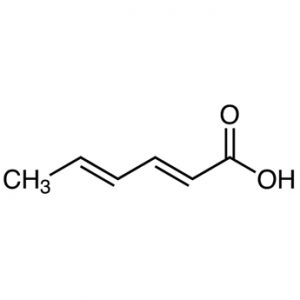
Description: Free-flowing, white, crystalline powder, with a characteristic odor.
Properties: Solubility 1 g in 400 mL water, 10 mL alcohol or 19mL propylene glycol; melting point 135°C; density 1.2 g/cc.
Incompatibilities: Bases, oxidizing agents, and reducing agents. Some functionality is lost in the presence of nonionic surfactants.
Uses: As a preservative.
Multiple choice questions:
1.Materials that prevent the initiation and growth of microorganisms in products are known as
a)antioxidants
b)preservatives
c)chelating agents
d)buffers
2.Which of the following are examples of preservatives?
a)Phenethyl Alcohol
b)Potassium Sorbate
c)Propylene Glycol
d)All of these
3.What are the synonyms of Alcohol?
a)Ethanol
b)Ethyl alcohol
c)Grain alcohol
d)All of these
4.Which of the following are physical properties of Benzalkonium chloride?
a)White to yellow-white powder
b)White crystalline odorless and tasteless powder
c)Colorless liquid with a rose-like odor
d)Colorless to light pink
5.H3BO3 is molecular formula of
a)Alcohol
b)Benzalkonium chloride
c)Boric acid
d)Sorbic acid
6.Butylated Hydroxyanisole (BHA) is used as
a)antioxidant
b)preservative
c)buffer
d)a and b
7.Butylparaben is used as
a)Antimicrobial preservative for liquid
b)Antimicrobial preservative for semi-solid preparations
c)Both of these
d)None of these
8.C8H8O3 is molecular formula of
a)Methylparaen
b)Phenol
c)Phenethyl Alcohol
d)Potassium Sorbate
9.Phenol shows incompatibility with
a)camphor
b)menthol
c)acetanilide
d)all of these
10.Phenylethyl Alcohol is used as
a)antioxidant
b)antimicrobial preservative
c)buffer
d)chelating agent
11.Potassium sorbate has molecular formula
a)C6H8O2
b)C10H12O3
c)C6H6O
d)C6H7KO2
12.1,2-Propanediol is chemical name of
a)Sorbic acid
b)Propylparaben
c)Propylene glycol
d)Potassium sorbate
13.Propylparaben is
a)Very poorly soluble in water
b)Highly soluble in water
c)soluble in both water as well as organic solvents
d)partially soluble in organic solvent
14.Synonym of Sorbic Acid is/are
a)2,4-hexadienoic acid
b)2-propenylacrylic acid
c)both of these
d)none of these
15.Which of the following are physical properties of Sorbic acid?
a)Free-flowing powder
b)White, crystalline powder
c)A characteristic odor
d)All of these
Solutions:
- b)preservatives
- d)All of these
- d)All of these
- a)White to yellow-white powder
- c)Boric acid
- d)a and b
- c)Both of these
- a)Methylparaen
- d)all of these
- b)antimicrobial preservative
- d)C6H7KO2
- c)Propylene glycol
- a)Very poorly soluble in water
- c)both of these
- d)All of these
References:
- Remington Essential of Pharmaceutics, 1st edition 2013, page no. 686, 687,698,696,697,698,699 ,700.
- Raymond C Rowe Handbook of Pharmaceutical Excipients, 10th edition 2009, page no. 17-19, 56-58, 68-70, 78-81, 441-445, 485-487, 490-491, 579-581, 592-594, 596-598, 672-675.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
The post Preservatives, Types of Preservatives, Properties of Preservatives and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>