The post Suspensions: Interfacial Properties of Suspended Particles and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>INTERFACIAL PROPERTIES OF SUSPENDED PARTICLES :
- Work must be done to reduce a solid to small particles and disperse them in a continuous medium.
- The large surface area of the particles that results from the comminution is associated with a surface free energy that makes the system thermodynamically unstable, by which we mean that the particles are highly energetic and tend to regroup in such a way as to decrease the total area and reduce the surface free energy.
- The particles in a liquid suspension therefore tend to flocculate, that is, to form light, fluffy conglomerates that are held together by weak van der Waals forces.
- Under certain conditions—in a compacted cake, for example—the particles may adhere by stronger forces to form what are termed aggregates.
- Caking often occurs by the growth and fusing together of crystals in the precipitates to produce a solid aggregate.
- The formation of any type of agglomerate, either floccules or aggregates, is taken as a measure of the system’s tendency to reach a more thermodynamically stable state.
- An increase in the work, W, or surface free energy, ΔG, brought about by dividing the solid into smaller particles and consequently increasing the total surface area, ΔA, is given by ΔG = γSL . ΔA.
where γSL is the interfacial tension between the liquid medium and the solid particles.
- To approach a stable state, the system tends to reduce the surface free energy; equilibrium is reached when ΔG = 0. This condition can be accomplished, as seen from equation .
- By a reduction of interfacial tension, or it can be approached by a decrease of the interfacial area.
- The latter possibility, leading to flocculation or aggregation, can be desirable or undesirable in a pharmaceutical suspension.
- The interfacial tension can be reduced by the addition of a surfactant but cannot ordinarily be made equal to zero.
- A suspension of insoluble particles, then, usually possesses a finite positive interfacial tension, and the particles tend to flocculate.
- The forces at the surface of a particle affect the degree of flocculation and agglomeration in a suspension.
- Forces of attraction are of the London–van der Waals type; the repulsive forces arise from the interaction of the electric double layers surrounding each particle.
- The formation of the electric double layer deals with interfacial phenomena.
- The potential energy of two particles is plotted as a function of the distance of separation.
- When the repulsion energy is high, the potential barrier is also high, and collision of the particles is opposed.
- The system remains deflocculated.
- When sedimentation is complete, the particles form a close-packed arrangement with the smaller particles filling the voids between the larger ones.
- Those particles lowest in the sediment are gradually pressed together by the weight of the ones above; the energy barrier is thus overcome, allowing the particles to come into close contact with each other.
- To resuspend and redisperse these particles, it is again necessary to overcome the high-energy barrier.
- Because this is not easily achieved by agitation, the particles tend to remain strongly attracted to each other and form a hard cake.
- When the particles are flocculated, the energy barrier is still too large to be surmounted, and so the approaching particle resides in the second energy minimum, which is at a distance of separation of perhaps 1000 to 2000 Å.
- This distance is sufficient to form the loosely structural flocs.
- These concepts evolve from (DLVO) theory for the stability of lyophobic sols.
- To summarize, flocculated particles are weakly bonded, settle rapidly, do not form a cake, and are easily resuspended.
- Deflocculated particles settle slowly and eventually form a sediment in which aggregation occurs with the resultant formation of a hard cake that is difficult to resuspend.
Multiple choice questions (MCQs)
1.Pharmaceutical suspension are generally
a)Flocculated
b)De flocculated
c)Both
d)None
2.Rate of sedimentation is high in
a)Flocculated
b)De flocculated
c)Both
d)None
3.Cake formation is characteristic feature of
a)Flocculated
b)De flocculated
c)Thixotropic suspension
d)Structured suspension
4.For ideal suspension, the sedimentation volume should be
a)Zero
b)Equal to one
c)Less than one
d)More than one
5.If particle size is 1-100µm then
a)Coarse dispersion
b)Colloidal dispersion
c)Flocculated
d)De flocculated
6.In dilute suspension the concentration of solid should be
a)2-10%
b)50%
c)5%
d)30%
7.The bioavailability of flocculated suspension is
a)Relatively high
b)Comparatively less
c)Both a and b
d)None of the above
8.In suspension, if particle size of suspended particle decrease so
a)Surface free energy decrease
b)Surface area decrease
c)Surface free energy increase
d)System becomes thermodynamically stable
9.As per sedimentation theory, if particle size of suspended particle decrease so sedimentation rate decrease. So what would be the effect on absorption of drug when taken orally?
a)Absorption increase
b)Absorption decrease
c)No effect
d)Both a and b
10.The sedimentation volume have a range from
a)5-6
b)2-4
c)3-5
d)0-1
11.A suspension is formed from uniform particles of solid, of diameter 10 Mm, suspended in a solvent. What is the best description of this system?
a)Monodisperse and coarse
b)Monodisperse and colloidal
c)Polydisperse and coarse
d)Polydisperse and colloidal
12.Which one of the following dispersions does not have liquid continuous phase?
a)Nanosuspension
b)Microemulsion
c)Gel
d)Foam
13.Following is not a mechanism for the separation of a physically unstable suspension of magnesium hydroxide in water?
a)Flocculation
b)Aggregation
c)Ostwald ripening
d)Hydrolysis
14.Brownian movement of particle in suspension cause
a)Assist sedimentation
b)Promote sedimentation
c)Prevent sedimentation
d)Increase sedimentation rate
15.Suspended particles become flocculated in a suspension, because
a)Particles are closely packed
b)Attractive forces between particles are appreciable
c)Repulsive forces between particles are appreciable
d)Vehicle rejects the particles
Solution:
- a)Flocculated
- a)Flocculated
- b)De flocculated
- c)Less than one
- c)Flocculated
- a)2-10%
- b)Comparatively less
- c)Surface free energy increase
- a)Absorption increase
- d)0-1
- a)Monodisperse and coarse
- c)Gel
- d)Hydrolysis
- c)Prevent sedimentation
- b)Attractive forces between particles are appreciable
References:
1. GAURAV KUMAR JAIN – THEORY & PRACTICE OF PHYSICAL PHARMACY, 1st edition 2012 Elsevier, page no. 204-206.
2. Martins Physical Pharmacy, 6th edition 2011, page no. 747-750.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
The post Suspensions: Interfacial Properties of Suspended Particles and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>The post Suspensions: Sedimentation of flocculated particles, sedimentation parameters and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>When sedimentation is studied in flocculated systems, it is observed that the flocs tend to fall together, producing a distinct boundary between the sediment and the supernatant liquid. The liquid above the sediment is clear because even the small particles present in the system are associated with the flocs. Such is not the case in deflocculated suspensions having a range of particle sizes, in which, in accordance with Stokes’s law, the larger particles settle more rapidly than the smaller particles. No clear boundary is formed (unless only one size of particle is present), and the supernatant remains turbid for a considerably longer period of time. Whether the supernatant liquid is clear or turbid during the initial stages of settling is a good indication of whether the system is flocculated or deflocculated, respectively. The initial rate of settling of flocculated particles is determined by the floc size and the porosity of the aggregated mass. Subsequently, the rate depends on compaction and rearrangement processes within the sediment. The term subsidence is sometimes used to describe settling in flocculated systems.
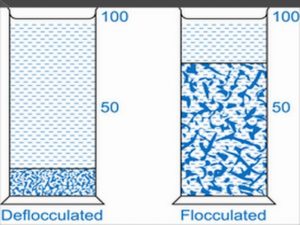
Fig 1 – Sedimentation in flocculated and deflocculated suspension(taken from formulation and bioavailability parameters of pharmaceutical suspension innovareacademics.in)
SEDIMENTATION PARAMETERS:
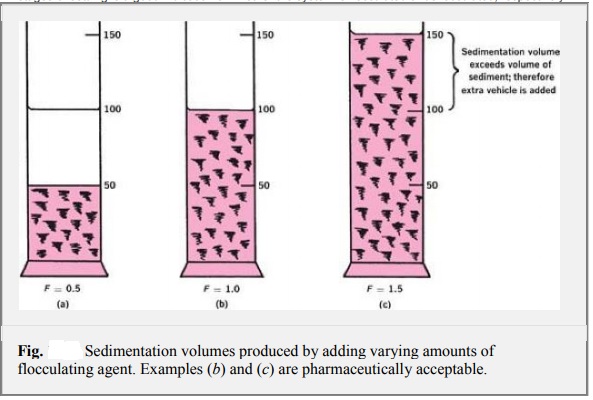
Two useful parameters that can be derived from sedimentation (or, more correctly, subsidence) studies are sedimentation volume, V, or height, H, and degree of flocculation. The sedimentation volume, F, is defined as the ratio of the final, or ultimate, volume of the sediment, Vu, to the original volume of the suspension, Vo, before settling. Thus, F = Vu/ Vo
The sedimentation volume can have values ranging from less than 1 to greater than 1.
F is normally less than 1, and in this case, the ultimate volume of sediment is smaller than the original volume of suspension. If the volume of sediment in a flocculated suspension equals the original volume of suspension, then F = 1. Such a product is said to be in flocculation equilibrium and shows no clear supernatant on standing. It is therefore pharmaceutically acceptable. It is possible for F to have values greater than 1, meaning that the final volume of sediment is greater than the original suspension volume. This comes about because the network of flocs formed in the suspension is so loose and fluffy that the volume they are able to encompass is greater than the original volume of suspension. This situation is illustrated in Figure c, in which sufficient extra vehicles have been added to contain the sediment(F = 1.5).
The sedimentation volume gives only a qualitative account of flocculation . A more useful parameter for flocculation is β, the degree of flocculation. If we consider a suspension that is completely deflocculated, the ultimate volume of the sediment will be relatively small. Writing this volume as V∞, based on equation F ∞ = V∞/V o Where F = sedimentation volume of the deflocculated, or peptized, suspension.
Substituting equations β = Vu/V o V∞/V o β = Vu/ V∞¢
The degree of flocculation, β, is therefore defined as the ratio of
F to F∞, β = F/ F∞.
The degree of flocculation is a more fundamental parameter than F because it relates the volume of flocculated sediment to that in a deflocculated system. We can therefore say that β= ultimate sediment volume of flocculated suspension ultimate sediment volume of deflocculated suspension.
Multiple choice questions (MCQs)
1.The particle size in suspension is
a)less than 103nm
b)102nm
c)greater than 103
d)10 nm
2.Suspension is
a)Heterogenous
b)Coarse dispersion
c)Both a and b
d)None
3.Which suspension is more stable for a short duration of time period?
a)Deflocculated
b)Flocculated
c)Both a and b
d)None
4.In ideal suspension particle should be
a)Non dispersible
b)Re dispersed
c)Forms cake
d)Should settle down
5.Stoke’s law is related to
a)Syrup
b)Suspension
c)Emulsion
d)None
6.Which suspension have more void space?
a)Flocculated
b)Deflocculated
c)Both a and b
d)None
7.____ is a zwitter ionic surfactant
a)SLS
b)Span
c)Lecithin
d)None
8.Suspension follow ____ kinetic
a)Zero order
b)Apparent zero
c)First order
d)Second order
9.HLB value of SLS is
a)10
b)40
c)12
d)None
10.____ dosage forms are uniform dispersion of insoluble solid or immiscible liquid in liquid phase
a)Monophasic liquid
b)Biphasic liquid
c)Oral liquids
d)All of the above
11.Which of the following internal phase present in the suspension?
a)Finely divided insoluble solids
b)Immiscible liquid
c)Water
d)Oil
12.Which of the following is a heterogenous liquid dosage form?
a)Solutions
b)Suspensions
c)Syrups
d)All of the above
13.Suspensions are
a)Biphasic
b)Heterogenous
c)Thermodynamically unstable
d)All of the above
14.The fine insoluble particles in suspensions are termed as
a)Internal phase
b)Discontinuous phase
c)Dispersed phase
d)All of the above
15.All are disadvantages of suspensions, except
a)Poor dose accuracy
b)Easy to swallow
c)Slower absorption than solutions
d)Aggregate formation
Solutions:
- c) greater than 103
- c)Both a and b
- a)Deflocculated
- b)Re dispersed
- b)Suspension
- a) Flocculated
- c) Lecithin
- b)Apparent zero
- b)40
- b)Biphasic liquid
- a)Finely divided insoluble solids
- b)Suspensions
- d)All of the above
- d)All of the above
- b)Easy to swallow
References:
1. GAURAV KUMAR JAIN – THEORY & PRACTICE OF PHYSICAL PHARMACY, 1st edition 2012 Elsevier, page no. 210-219.
2. Martins Physical Pharmacy, 6th edition 2011, page no. 751-752.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
The post Suspensions: Sedimentation of flocculated particles, sedimentation parameters and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>