The post Comparative observational studies –Cohort Study | Pharmacovigilance Notes and Lecture appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>However, they remain important because many questions can be efficiently answered by these methods and sometimes they are the only methods available. The objective of most clinical studies is to determine one of the following—prevalence, incidence, cause, prognosis, or effect of treatment; it is therefore useful to remember which type of
study is most commonly associated with each objective
While an appropriate choice of study design is vital, it is not sufficient. The hallmark of good research is the rigor with which it is conducted. Every published study should contain sufficient information to allow the reader to analyse the data with reference to these key points.
COHORT STUDIES
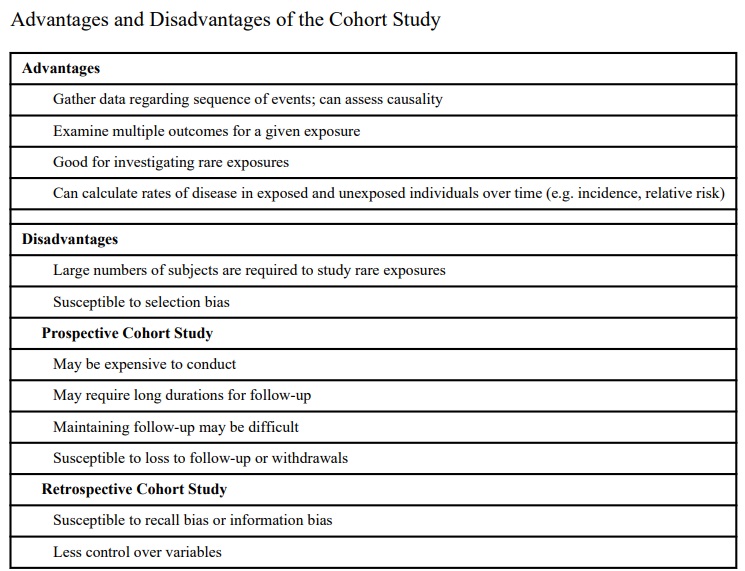
These are the best method for determining the incidence and natural history of a condition. The studies may be prospective or retrospective and sometimes two cohorts are compared.
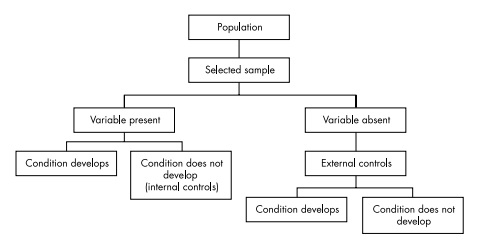
Prospective cohort studies A group of people is chosen who do not have the outcome of interest (for example, myocardial infarction). The investigator then measures a variety
of variables that might be relevant to the development of the condition. Over a period of time the people in the sample are observed to see whether they develop the outcome of interest (that is, myocardial infarction).
In single cohort studies those people who do not develop the outcome of interest are used as internal controls.
Where two cohorts are used, one group has been exposed to or treated with the agent of interest and the other has not, thereby acting as an external control.
Retrospective cohort studies These use data already collected for other purposes. The methodology is the same but the study is performed posthoc. The cohort is
“followed up” retrospectively. The study period may be many years but the time to complete the study is only as long as it takes to collate and analyse the data
How to run a cohort study
If the data are readily available then a retrospective design is the quickest method. If high quality, reliable data are not available a prospective study will be required.
The first step is the definition of the sample group. Each subject must have the potential to develop the outcome of interest (that is, circumcised men should not be included in a cohort designed to study paraphimosis). Furthermore, the sample population must be representative of the general population if the study is primarily looking at the incidence and natural history of the condition (descriptive).
If however the aim is to analyse the relation between predictor variables and outcomes (analytical) then the sample should contain as many patients likely to develop the outcome as possible, otherwise much time and expense will be spent collecting information of little value.
Each variable studied must be accurately measured. Variables that are relatively fixed, for example, height need only be recorded once. Where change is more probable, for
example, drug misuse or weight, repeated measurements will be required.
To minimise the potential for missing a confounding variable all probable relevant variables should be measured. If this is not done the study conclusions can be readily criticised. All patients entered into the study should also be followed up for the duration of the study. Losses can significantly affect the validity of the results. To minimise this as much information about the patient (name, address, telephone, GP, etc) needs to be recorded as soon as the patient is entered into the study.
Regular contact should be made; it is hardly surprising if the subjects have moved or lost interest and become lost to follow up if they are only contacted at 10 year intervals!
Beware, follow up is usually easier in people who have been exposed to the agent of interest and this may lead to bias.
The post Comparative observational studies –Cohort Study | Pharmacovigilance Notes and Lecture appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>The post Pharmacovigilance Methods- Active Surveillance | Sentinel sites, Drug event monitoring and Registries appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>Active Surveillance
Active surveillance, in contrast to passive surveillance, pursues to determine the particular number of adverse events through a constant pre-organized process. In common, it is more achievable to acquire wide-ranging data on discrete adverse event reports through an active surveillance system than through a passive reporting system.
a) Sentinel sites
Active surveillance can be attained by revising medical records or questioning patients and/or physicians in a section of sentinel sites to guarantee that comprehensive and precise data on reported adverse events are collected from these sites. The selected sites can deliver information, such as data from specific patient subgroups, which would not be accessible in a passive spontaneous reporting system.
The major weaknesses of sentinel sites comprise difficulties with selection bias, small numbers of patients and augmented costs. Active surveillance with sentinel sites is most effective for those medicines used primarily in institutional settings such as hospitals, nursing homes and haemodialysis centers.
Institutional settings may use certain medicinal products more commonly and can deliver an arrangement for enthusiastic reporting. Intensive monitoring of sentinel sites
can also be supportive in recognizing risks among patients taking orphan medicines.
b) Drug Event Monitoring- can be divided into i) Medicine event monitoring ii) Cohort event monitoring
i) Medicine event monitoring
This is a process of active Pharmacovigilance surveillance. Studies using this process are cohort-based and prospective and observational. For medication event monitoring, patients can be acknowledged from electronic or automated health insurance claims. A single prescription or a series might be composed over the period of monitoring. A follow-up questionnaire can then be sent to each prescribing physician or patient at pre-specified intervals to acquire outcome data.
Requests for data on patient demographics, indication for treatment, duration of therapy, dosage, clinical events, reasons for termination and applicable past history can be
involved in the questionnaires. The restrictions of medicine event monitoring can comprise the poor physician and patient reply rates.
ii) Cohort Event monitoring
In a cohort study, a population at risk for the disease (or event) is monitored over time to record the occurrence of the disease (or event). Information on exposure status is
accessible during the follow-up period for each patient. A patient might be exposed to a medicine at one time during follow-up, but not exposed at another time. Meanwhile the
population exposure during follow-up is acknowledged, incidence rates can be calculated.
In many cohort studies concerning medicine exposure, appraisal cohorts of interest are selected on the basis of medicine use and monitored over time. Cohort studies are useful when there is a requisite to know the incidence rates of adverse events in addition to the relative risks. Multiple adverse events can also be scrutinized using the similar data source in a cohort study. Conversely, it can be problematic to recruit adequate numbers of patients who are exposed to the medicine of interest or to study very
rare outcomes.
Similar to case-control studies, patients in cohort studies can be recognized from large automated databases or from data collected precisely for the study at hand. In addition, cohort studies can be used to scrutinize safety issues in special populations through oversampling of these patients or by stratifying the cohort if adequate numbers of patients are included. There are numerous automated databases obtainable for pharmacoepidemiological studies. They consist of databases that contain automated medical records or
automated accounting/billing systems.
Databases that are fashioned from accounting/billing systems might be connected to pharmacy claims and medical claims databases. These datasets may contain millions of patients. Subsequently, they are fashioned for administrative or billing purposes; they might not have all the detailed and precise information needed for some research, such as authenticated diagnostic information or laboratory data. Even though medical records can be used to establish and authenticate test results and medical diagnoses, one should know about the privacy and privacy regulations that apply to patient medical records
c) Registries
A registry is a list of patients presenting with the identical representative(s). This representative can be a disease (disease registry) or a specific exposure (medicine registry).
Both types of registrations, which vary only by the type of patient data of interest, can gather a cordless of information using standardized questionnaires in a prospective fashion. Disease registries, such as registries for blood dyscrasias, severe cutaneous reactions, or congenital malformations can help to gather data on medicine exposure and other factors related to a clinical condition.
A disease registry might also be used as a veil for a case control study associating the medicine exposure of cases recognized from the registry with controls selected either from patients with another condition within the registry, or from patients outside the registry.
Exposure (medicine) registries address populations exposed to the medicines of interest to govern if a medicine has a distinct influence on this group of patients. Some exposure (medicine) registries address drug exposures in specific populations, such as pregnant women.
Patients can be followed over time and included in a cohort study to collect data on adverse events using standardized questionnaires. Single cohort studies can quantity incidence, but, without a comparison group, cannot deliver proof of association. This type of registry can be very valuable when examining the safety of an orphan medicine indicated for a specific condition.
Customary epidemiological methods are a key constituent in the evaluation of adverse events. There are numerous of observational study designs that are valuable in
validating signals from spontaneous reports, case series or medicine event monitoring. The most imperative of these designs is cross-sectional studies, case-control studies and
cohort studies
Reference:
http://z.umn.edu/INNOVATIONS 2015, Vol. 6, No. 1, Article 189
Notes compiler
Dr Vivek Jain
The post Pharmacovigilance Methods- Active Surveillance | Sentinel sites, Drug event monitoring and Registries appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>