The post NALORPHINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>IUPAC nomenclature
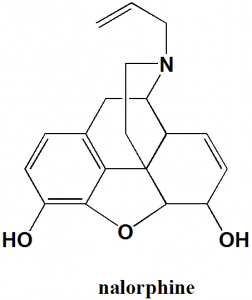
17-allyl-7,8-didehydro-4,5α-epoxymorphinan-3,6α-diol.
Classification
- Nalorphine is a mixed opioid agonist-antagonist.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 311.4 g/mol |
| 2 | Physical appearance | Crystals from diethyl ether |
| 3 | Melting point | 208.5°C |
| 4 | Solubility | Slightly soluble in water; sparingly soluble in ether |
| 5 | Octanol/water partition coefficient | 1.86 |
| 6 | Presence of ring | Piperidine, cyclohexene, tetrahydrofuran, phenyl |
| 7 | Number of chiral centers | 5 |
Mechanism of Action
Nalorphine acts on to opioid receptors:
i. On mu-receptors it shows antagonistic effects
ii. On kappa receptors, it shows high-efficacy agonistic effects.
Structure Activity Relationship
SAR for opiates can be summarized as follows:
- Replacement of phenolic hydroxyl into –OCH3/-OC2H5 will make the drug less analgesic and cough suppression will also takes place.
- Replacement of alcoholic hydroxyl with –OCH3 makes the compound 5 times more active.
- Replacement of alcoholic hydroxyl with -OC2H5 makes the compound 2.4 times more active than drug.
- Replacement of alcoholic hydroxyl with –OCOCH3 will also activate the compound by 4.2 times.
- Replacement of alcoholic hydroxyl with ketone group inactivates the compound and makes it lesser active.
- By hydrogenation of alicyclic unsaturated linkage, activity increases by 1.2 times.
- On replacement of the methyl group from tertiary nitrogen by hydrogen atom, activity decreases.
- On replacement of N-CH3 by NCH2CH2Ph, activity increases by 14 times.
- When the methyl group of tertiary nitrogen replaced by N-allyl/methallyl/propyl, the compound so formed acts like the Drug antagonist.
- When the methyl group of tertiary nitrogen replaced by N(CH3)2 Cl– , compound have curare action and it do not possesses any analgesic activity.
Method of synthesis
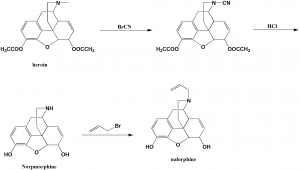
i. Heroin is processed with cyanogens bromide to give N-cyano derivative.
ii. The N-cyano derivative is hen hydrolyzed by a solution of hydrochloric acid to transform into N-demethylated morphine which is also called as normorphine.
iii. The secondary amine group of normorphine undergoes alkylation with allylbromide to give the desired nalorphine.[1]
Therapeutic Uses
Nalorphine is used for:
- To reverse the opioid overdose
- Challenge test to determine opioid dependence
Side Effects
Side effects Nalorphine are:
- Anxiety
- Hallucinations
- Confusions
- Dysphoria
MCQs
Q.1 What can be the correct IUPAC nomenclature of Nalorphine?
a) 17-allyl-7,8-didehydro-4,5α-epoxymorphinan-3,6ß-diol
b) N-phenyl-N-[1-(2-phenylethyl)piperidin-4-yl]propanamide
c) N-[1-(2-phenylethyl)piperidin-4-yl]propanamide
d) 17-allyl-7,8-didehydro-4,5α-epoxymorphinan-3,6α-diol
Q.2 Which amongst the following statements is/are incorrect related to the SAR of opiates?
I. On replacement of the methyl group from tertiary nitrogen by hydrogen atom, activity increases.
II. On replacement of N-CH3 by NCH2CH2Ph, activity decreases by 14 times.
III. When the methyl group of tertiary nitrogen replaced by N-allyl/methallyl/propyl, the compound so formed acts like the Drug antagonist
a) I, II
b) I, II, III
c) III
d) I
Q.3 Nalorphine can be synthesized from alkylation of ___________ by allylbromide?
a) Morphine
b) Normorphine
c) Codeine
d) Pentazocine
Q.4 Side effects of drug Nalorphine is/are?
a) Anxiety
b) Hallucinations
c) Confusions
d) All of the above
Q.5 Match the following drugs with their correct molecular weight.
| i. Nalorphine | A. 311.4 gm/mol |
| ii. Phenacemide | B. 184.49 gm/mol |
| iii. Loxapine | C. 178.19 gm/mol |
| iv. Enflurane | D. 327.8 gm/mol |
a) i-A, ii-D, iii-C, iv-B
b) i-B, ii-A, iii-D, iv-C
c) i-A, ii-C, iii-D, iv-B
d) i-D, ii-C, iii-A, iv-B
Q.6 An example of drug from class Mixed-opioid antagonist is?
a) Gabapentin
b) Nalorphine
c) Levallorphan
d) Secobarbital
Q.7 The type of ring system found in the structure of Nalorphine is?
a) Piperidine
b) Furan
c) Cyclohexane
d) All of the above
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-d
2-a
3-b
4-d
5-c
6-b
7-d
REFERENCES
[1] Vardanyan R, Hruby V. Synthesis of essential drugs. Elsevier; 2006 Mar 10.The post NALORPHINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>The post THIOTEPA Synthesis, SAR, MCQ and Chemical Structure appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>IUPAC nomenclature
1,1′,1′′-Phosphorothioyltriaziridine
Classification
Thiotepa falls under the category of ethylenimine alkalyting agents.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 189.22 g/mol |
| 2 | Appearance | White crystalline solid |
| 3 | Melting point | 51.5°C |
| 4 | Solubility | 19 mg per 100 ml at NTP |
| 5 | Octanol water partition coefficient | 0.53 |
| 6 | Presence of ring | Aziridine rings |
Mechanism of Action
- After administration, thiotepa converts into ethylenimine groups.
- Ethylenimine groups attaches with N7 position of guanine base pair of DNA
- This will induce the cross linkages between the ds-DNA.
- This will further interferes with the processes such as DNA replication and transcription
- This will result in the inhibition of the cell growth and apoptosis of the cell. [1]
Structural Activity Relationship
- Replacement of the sulfur atom by nitrogen will lower the toxicity.
- 2-chloroethyl group is essential for the activity as the aziridine cation is formed by this only. Aziridine cation will attach with the alkylates of the DNA later.
- Binding with the amino group will increase the oral route availability of the drug
- The introduction of the substituted phenyl group will also increases the oral route availability of the drug.
- Aromatic ring introduction will increase the stability of the drug.
- Aromatic ring will further increase the distribution of the drug throughout the body.
- Benzimidazole ring can provide the local and faster action of the drug.
- Benzimidazol will further decrease the half life of compound. [2]
Methods of Synthesis
- Ethylineimine is reacted with thiophosphoryl chloride in presence of triethyamine in dry benzene as a solvent.
Therapeutic Uses
- Breast cancer
- Ovarian cancer
- Hodgkin’s lymphomas
- Non-Hodgkin’s lymphomas
- Superficial tumors of bladder
Side Effects
- Most common side effect is low blood count
- Other side effects includes nausea, vomiting, mouth sores, skin allergies and bladder irritation.
MCQs
Q.1 Correct IUPAC name for thiotepa can be?
a) 1,1′,2-Phosphorothioyltriaziridine
b) 1,2,2′-Phosphorothioyltriaziridine
c) 1,1′,1′′-Phosphorothioyltriaziridine
d) 1,2,3-Phosphorothioyltriaziridine
Q.2 Predict the incorrect statement related to Thiotepa
a) It is highly toxic
b) It is seldom used now
c) It needs to be converted into its active intermediate to show its effects
d) It has aziridine rings in it
Q.3 Oral rout availability of Thiotepa can be increased through
a) Binding with amino group
b) Binding with benzimidazole ring
c) Binding with substituted phenyl group
d) Both a) and c)
Q.4 Thiotepa shows its effect through
a) Alkylation of guanine base pair of DNA
b) Altering the cell membrane structure of cancerous cells
c) Inhibiting the mitochondria to produce energy
d) Rupturing the nuclear membrane
Q.5 Classification of Thiotepa is
a) Nitrogen mustard alkalyting agent
b)Triazine alkalyting agent
c) Ethylinimine alkalyting agent
d) Nitrosoureas alkalyting agent
Q.6 An important side effect of Thiotepa is
a) Rhabdomyosarcoma
b) Ovarian cancer
c) Low blood cell count
d) Bladder cell apoptosis
Q.7 Number of aziridine rings present in Thioptepa?
a) 1
b) 2
c) 3
d) 0
For More Standard and Quality Question Bank you can Join Our Test Series Programme for GPAT, NIPER JEE, Pharmacist Recruitment Exam, Drug Inspector Recruitment Exams, PhD Entrance Exam for Pharmacy: Click Here
ANSWERS
1-c
2-c
3-d
4-a
5-c
6-c
7-c
References
[1] Van Maanen MJ, Smeets CJ, Beijnen JH. Chemistry, pharmacology and pharmacokinetics of N, N′, N′′-triethylenethiophosphoramide (ThioTEPA). Cancer treatment reviews. 2000 Aug 1;26(4):257-68. [2] Pires J, Kreutz OC, Suyenaga ES, Perassolo MS. PHARMACOLOGICAL PROFILE AND STRUCTURE-ACTIVITY RELATIONSHIP OF ALKYLATING AGENTS USED IN CANCER TREATMENT. [3] Wilson CO, Beale JM, Block JH. Wilson and Gisvold’s textbook of organic medicinal and pharmaceutical chemistry. Baltimore, MD: Lippincott Williams & Wilkins,; 2011: pp.360-362.The post THIOTEPA Synthesis, SAR, MCQ and Chemical Structure appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>