NALORPHINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
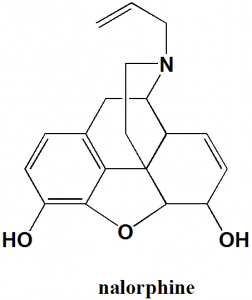
Nalorphine
IUPAC nomenclature
17-allyl-7,8-didehydro-4,5α-epoxymorphinan-3,6α-diol.
Classification
- Nalorphine is a mixed opioid agonist-antagonist.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 311.4 g/mol |
| 2 | Physical appearance | Crystals from diethyl ether |
| 3 | Melting point | 208.5°C |
| 4 | Solubility | Slightly soluble in water; sparingly soluble in ether |
| 5 | Octanol/water partition coefficient | 1.86 |
| 6 | Presence of ring | Piperidine, cyclohexene, tetrahydrofuran, phenyl |
| 7 | Number of chiral centers | 5 |
Mechanism of Action
Nalorphine acts on to opioid receptors:
i. On mu-receptors it shows antagonistic effects
ii. On kappa receptors, it shows high-efficacy agonistic effects.
Structure Activity Relationship
SAR for opiates can be summarized as follows:
- Replacement of phenolic hydroxyl into –OCH3/-OC2H5 will make the drug less analgesic and cough suppression will also takes place.
- Replacement of alcoholic hydroxyl with –OCH3 makes the compound 5 times more active.
- Replacement of alcoholic hydroxyl with -OC2H5 makes the compound 2.4 times more active than drug.
- Replacement of alcoholic hydroxyl with –OCOCH3 will also activate the compound by 4.2 times.
- Replacement of alcoholic hydroxyl with ketone group inactivates the compound and makes it lesser active.
- By hydrogenation of alicyclic unsaturated linkage, activity increases by 1.2 times.
- On replacement of the methyl group from tertiary nitrogen by hydrogen atom, activity decreases.
- On replacement of N-CH3 by NCH2CH2Ph, activity increases by 14 times.
- When the methyl group of tertiary nitrogen replaced by N-allyl/methallyl/propyl, the compound so formed acts like the Drug antagonist.
- When the methyl group of tertiary nitrogen replaced by N(CH3)2 Cl– , compound have curare action and it do not possesses any analgesic activity.
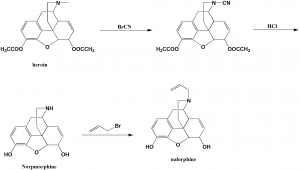
Method of synthesis
i. Heroin is processed with cyanogens bromide to give N-cyano derivative.
ii. The N-cyano derivative is hen hydrolyzed by a solution of hydrochloric acid to transform into N-demethylated morphine which is also called as normorphine.
iii. The secondary amine group of normorphine undergoes alkylation with allylbromide to give the desired nalorphine.[1]
Therapeutic Uses
Nalorphine is used for:
- To reverse the opioid overdose
- Challenge test to determine opioid dependence
Side Effects
Side effects Nalorphine are:
- Anxiety
- Hallucinations
- Confusions
- Dysphoria
MCQs
Q.1 What can be the correct IUPAC nomenclature of Nalorphine?
a) 17-allyl-7,8-didehydro-4,5α-epoxymorphinan-3,6ß-diol
b) N-phenyl-N-[1-(2-phenylethyl)piperidin-4-yl]propanamide
c) N-[1-(2-phenylethyl)piperidin-4-yl]propanamide
d) 17-allyl-7,8-didehydro-4,5α-epoxymorphinan-3,6α-diol
Q.2 Which amongst the following statements is/are incorrect related to the SAR of opiates?
I. On replacement of the methyl group from tertiary nitrogen by hydrogen atom, activity increases.
II. On replacement of N-CH3 by NCH2CH2Ph, activity decreases by 14 times.
III. When the methyl group of tertiary nitrogen replaced by N-allyl/methallyl/propyl, the compound so formed acts like the Drug antagonist
a) I, II
b) I, II, III
c) III
d) I
Q.3 Nalorphine can be synthesized from alkylation of ___________ by allylbromide?
a) Morphine
b) Normorphine
c) Codeine
d) Pentazocine
Q.4 Side effects of drug Nalorphine is/are?
a) Anxiety
b) Hallucinations
c) Confusions
d) All of the above
Q.5 Match the following drugs with their correct molecular weight.
| i. Nalorphine | A. 311.4 gm/mol |
| ii. Phenacemide | B. 184.49 gm/mol |
| iii. Loxapine | C. 178.19 gm/mol |
| iv. Enflurane | D. 327.8 gm/mol |
a) i-A, ii-D, iii-C, iv-B
b) i-B, ii-A, iii-D, iv-C
c) i-A, ii-C, iii-D, iv-B
d) i-D, ii-C, iii-A, iv-B
Q.6 An example of drug from class Mixed-opioid antagonist is?
a) Gabapentin
b) Nalorphine
c) Levallorphan
d) Secobarbital
Q.7 The type of ring system found in the structure of Nalorphine is?
a) Piperidine
b) Furan
c) Cyclohexane
d) All of the above
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-d
2-a
3-b
4-d
5-c
6-b
7-d