LEVORPHANOL Synthesis, SAR, MCQ, Structure, Chemical Properties and Therapeutic Uses
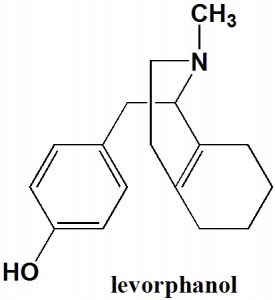
Levorphanol
IUPAC nomenclature
(1R,9R,10R)-17-Methyl-17-azatetracyclo[7.5.3.0¹,¹⁰.0²,⁷]heptadeca-2(7),3,5-trien-4-ol
Classification
- Levorphanol falls under category of opioid analgesics.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 257.37 g/mol |
| 2 | Physical appearance | Crystals |
| 3 | Melting point | 198-199°C |
| 4 | Solubility | 329 mg/L in water |
| 5 | Octanol/water partition coefficient | 3.11 |
| 6 | Presence of ring | Phenyl, piperidine |
| 7 | Number of chiral centers | 1 |
Mechanism of Action
Levorphanol is μ-agonist opioid. It acts on the receptors present in the periqueductal and periventruicular gray matter in brain as well as in the spinal cord and alters the transmission of the pain signals.
Structure Activity Relationship
SAR for opioid analogues can be summarized as follows:
- Replacement of phenolic hydroxyl into –OCH3/-OC2H5 will make the drug less analgesic and cough suppression will also takes place.
- Replacement of alcoholic hydroxyl with –OCH3 makes the compound 5 times more active.
- Replacement of alcoholic hydroxyl with -OC2H5 makes the compound 2.4 times more active than drug.
- Replacement of alcoholic hydroxyl with –OCOCH3 will also activates the compound by 4.2 times.
- Replacement of alcoholic hydroxyl with ketone group inactivates the compound and makes it lesser active.
- By hydrogenation of alicyclic unsaturated linkage, activity increases by 1.2 times.
- On replacement of the methyl group from tertiary nitrogen by hydrogen atom, activity decreases.
- On replacement of N-CH3 by NCH2CH2Ph, activity increases by 14 times.
- When the methyl group of tertiary nitrogen replaced by N-allyl/methallyl/propyl, the compound so formed acts like the Drug antagonist.
- When the methyl group of tertiary nitrogen replaced by N(CH3)2 Cl– , compound do not possesses any analgesic activity.
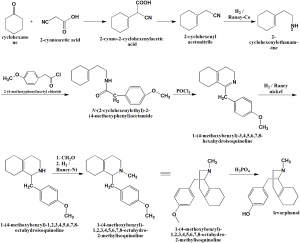
Method of synthesis
i. Cyclohexanone undergoes condensation with cyanoacetic acid; simultaneously decarboxylation occurs to form cyclohexenylacetonitrile.
ii. Reduction of the nitrile groupby hydrogen in presence of Raney cobalt gives 2-(1-cyclohexenyl)ethylamine.
iii. The above formed compound is acylated by 4-mehoxyphenylacetyl chloride to form the amide 2-(1-cyclohexenyl)ethyl-4-methoxyphenylacetamide.
iv. On cyclization using phosphorus oxychloride leads to formation of 1-(4-methoxybenzyl)-3,4,5,6,7,8-hexahydroquinolin.
v. The imine bond of last is hydrogenated in the presence of Raney nickel to form 1-(4-methoxybenzyl)-1,2,3,4,5,6,7,8-octahydroquinolin.
vi. Last undergoes cyclization and simultaneous demethylation to form levorphanol.[1]
Therapeutic Uses
Levorphanol is used for:
- Relieve moderate to severe pain
Side Effects
Side effects Levorphanol are:
- Headache
- Heartburn
- Stomach pain
- Dry mouth
- Sweating
- Difficulty urinating
- Vision problems
- Dizziness
- Nausea
- Vomiting
- Loss of appetite
- Weakness
- Rash
- Hives
- Agitation
- Hallucinations
- Decreased libido
- Swellings
- Difficulty in breathing
- Changes in heartbeat
- Hoarseness
MCQs
Q.1 “(1R,9R,10R)-17-Methyl-17-azatetracyclo[7.5.3.0¹,¹⁰.0²,⁷]heptadeca-2(7),3,5-trien-4-ol” is the IUPAC nomenclature of which drug?
a) Methotrexate
b) Letrozole
c) Triptorelin
d) Levorphanol
Q.2 Correct melting point of the drug Levorphanol is?
a) 235°C
b) 253°C
c) 198.5°C
d) 137.5°C
Q.3 Match the following with correct classifications of the drugs.
| i.Nafarelin | A. GnRH analogues |
| ii. 6-Mercaptopurin | B. ß-blockers |
| iii. Levorphanol | C. Folate antagonist |
| iv. Labetolol | D. Opioid analgesic |
a) i-A, ii-C, iii-D, iv-B
b) i-C, ii-A, iii-B, iv-D
c) i-D, ii-C, iii-A, iv-B
d) i-A, ii-D, iii-C, iv-B
Q.4 Mechanism of action of drug Levorphanol includes?
I. Alters transmission of pain signal.
II. Agonist for mu-opioid receptors
III. Decreases the fluidity of the lipid membrane.
IV. Activates calcium dependent ATPase in sarcoplasmic reticulum.
a) II, III, IV
b) I, II, IV
c) I, III, IV
d) I, II
Q.5 Correct sequence for True and False for the given statements related with the SAR of Inhaled anesthetics drugs?
- On replacement of the methyl group from tertiary nitrogen by hydrogen atom, activity increases.
- On replacement of N-CH3 by NCH2CH2Ph, activity increases by 14 times.
- When the methyl group of tertiary nitrogen replaced by N-allyl/methallyl/propyl, the compound so formed acts like the Drug antagonist.
- When the methyl group of tertiary nitrogen replaced by N(CH3)2 Cl– , compound do not possesses any analgesic activity
a) FTTT
b) TFTF
c) TFFF
d) TTTT
Q.6 Type of ring present in the structure of levorphanol?
a) Piperidine
b) Furan
c) Purine
d) Diazepine
Q.7 The drug levorphanol is used for?
a) Induction of anesthesia
b) Relieve moderate to severe pain
c) Treatment of panic attacks
d) All of the above
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-d
2-c
3-a
4-d
5-a
6-d
7-b