The post Surface and interfacial phenomenon: Electrical properties of interfaces and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>The existence of difference in electrical potential across a solid–liquid interface is demonstrated by the following phenomena:
Electrophoresis: Movement of dispersed particles through a liquid medium under the influence of an electric field.
Electro-osmosis: Movement of a liquid relative to a fixed solid under the influence of an electric field.
Streaming potential: Potential difference set up across a fixed porous plug of solid when a liquid is forced through it.
Sedimentation potential: Potential difference set up between the top and bottom of dispersion of solid particles in a liquid when particles settle under the influence of gravity.
The above-mentioned electrokinetic phenomenon across an interface indicates that there must be a particular distribution of charge near the interface. This distribution is referred to as the electrical double layer.
Electrical Double Layer:
Let us consider solid particles carrying positive charge in contact with an aqueous solution containing positive and negative ions. The positively charged solid surface will influence the distribution of ions in the nearby layers of the solution. Thus, negative ions will be attracted towards the solid surface and negative ions repelled away from it. The resulting effects create a diffuse layer of solution in which negative ions gradually decrease on moving away from the interface and positive ions gradually increase. This type of distribution is referred to as the electrical double layer.
Stern layer: Strong adsorption of oppositely charged ions to the surface of particle.
Gouy layer: Distribution of oppositely charged ions in the diffuse layer.
The distribution of ions will affect the potential at varying distances. Potential decreases linearly across the Stern layer (stern potential; potential at the boundary between Stern and Gouy layer) from the surface potential and then decreases comparatively slowly until it is zero at the edge of the Gouy layer.
A layer of liquid will also be adsorbed onto the solid particle (solvating layer).

Fig 1 – Idealized representation of the electrical double layer(taken from conceptual representation of the electric double layer Research gate)
This solvating layer is strongly held to the surface and its outer surface represents the boundary of relative movement between the solid and the liquid. The potential at this point is termed as Zeta potential.
- Increasing the amount of electrolytes or increasing the valency of the counterion (keeping the total concentration of the electrolyte constant) decreases the Stern and Zeta potentials owing to the decrease in thickness of the double layer.
- Zeta potential acts as an energy barrier for the stability of colloids and suspensions.
- Zeta potential determines the degree of repulsion between adjacent, similarly charged dispersed particles and therefore has practical applications in the stability of systems containing dispersed particles.
Multiple choice questions (MCQs)
1.Movement of dispersed particles through a liquid medium under the influence of an electric field is called as
a)Electrophoresis
b)Electro-osmosis
c)Streaming potential
d)Sedimentation potential
2.Movement of a liquid relative to a fixed solid under the influence of an electric field is known as
a)Electrophoresis
b)Electro-osmosis
c)Streaming potential
d)Sedimentation potential
3.Potential difference set up across a fixed porous plug of solid when a liquid is forced through it is known as
a)Electrophoresis
b)Electro-osmosis
c)Streaming potential
d)Sedimentation potential
4.Potential difference set up between the top and bottom of dispersion of solid particles in a liquid when particles settle under the influence of gravity is called
a)Electrophoresis
b)Electro-osmosis
c)Streaming potential
d)Sedimentation potential
5.The existence of difference in electrical potential across a solid–liquid interface is demonstrated by which of the following phenomena?
a)Electro-osmosis
b)Streaming potential
c)Sedimentation potential
d)All of the above
6.Which of the following is strong adsorption of oppositely charged ions to the surface of particle?
a)Stern layer
b)Gouy layer
c)Both of these
d)None of these
7.Which of the following is distribution of oppositely charged ions in the diffuse layer?
a)Stern layer
b)Gouy layer
c)Both of these
d)None of these
8.The distribution of ions will affect the potential at varying distances.
a)True
b)False
9.Potential decreases linearly across the Stern layer (stern potential; potential at the boundary between Stern and Gouy layer) from the surface potential and then decreases comparatively slowly until it is zero at the edge of the Gouy layer.
a)True
b)False
10.The solvating layer is strongly held to the surface and its outer surface represents the boundary of relative movement between the solid and the liquid. The potential at this point is termed as
a)Zeta potential
b)Nernst potential
c)Stern layer
d)All of these
11.Which of the following is correct statement?
a)Increasing the amount of electrolytes or increasing the valency of the counterion (keeping the total concentration of the electrolyte constant) decreases the Stern and Zeta potentials owing to the decrease in thickness of the double layer.
b)Zeta potential acts as an energy barrier for the stability of colloids and suspensions.
c)Zeta potential determines the degree of repulsion between adjacent, similarly charged dispersed particles and therefore has practical applications in the stability of systems containing dispersed particles.
d)All of the above
12.Immediately adjacent to the interface in electric double layer model is a region called
a)Tightly bound layer
b)Diffuse second layer
c)Nernst potential
d)Zeta potential
13.Excess negative ions are present in this region in electric double layer model
a)Tightly bound layer
b)Diffuse second layer
c)Nernst potential
d)Zeta potential
14.It is the potential of solid surface itself owing to the presence of potential determining ions
a)Tightly bound layer
b)Diffuse second layer
c)Nernst potential
d)Zeta potential
15.It is the potential observed at shear plane.
a)Tightly bound layer
b)Diffuse second layer
c)Nernst potential
d)Zeta potential
Solutions:
- a)Electrophoresis
- b)Electro-osmosis
- c)Streaming potential
- d)Sedimentation potential
- d)all of the above
- a)Stern layer
- b)Gouy layer
- a)True
- a)True
- a)Zeta potential
- d)All of the above
- a)Tightly bound layer
- b)Diffuse second layer
- c)Nernst potential
- d)Zeta potential
References:
1. GAURAV KUMAR JAIN – THEORY & PRACTICE OF PHYSICAL PHARMACY, 1st edition 2012 Elsevier, page no. 136-137.
2. Martins Physical Pharmacy, 6th edition 2011, page no. 705-707.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
The post Surface and interfacial phenomenon: Electrical properties of interfaces and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>The post Surface and interfacial phenomenon: Complex films and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>Insoluble Monomolecular Films: Insoluble amphiphilic compounds such as surfactants with very long hydrocarbon chains form films one molecule in thickness on water surfaces when the surfactant is dissolved in a volatile solvent and carefully injected onto the surface. The molecules are orientated at the surface in the same way as typical surfactants, i.e. with the hydrophobic group protruding into the air and the polar group acting as an anchor in the surface. The thickness of such films can be determined if the area of the film and the volume of the spreading liquid are known. The properties of the film can be studied using the film balance.
The surfactant is dissolved in a volatile solvent and is spread as a film on the surface of water (substrate). The volatile solvent evaporates, leaving behind the surfactant film over water. With the help of stationary and movable barrier, the area of the film is determined and the movable barrier is then forced to move towards the stationary barrier, thus compressing the film gradually. At each position, the area of the film (A) and the film pressure (S) are measured. The results are presented as plots of area of film against the film pressure (S = Jo – Jm, where Jo is the surface tension of water surface and Jm the surface tension of the film-covered surface), called S–A curves.
Gaseous films: In gaseous films, the adsorbed surfactant molecules do not adhere to each other laterally, and move freely around the interface. The charged groups repel one another in the aqueous solution as the droplet covered with the film moves closer to one another. In such films, there is only a gradual change in the surface pressure as the film is compressed. One example of a gaseous film is that formed by the anionic surfactant, sodium dodecyl sulphate.
Expanded films: Films formed by oleic acid are expanded. The hydrocarbon chains in oleic acid are less cohesive and less orderly packed due to the higher polarity and affinity for water. The presence of branched and bent-shaped hydrocarbon chains, bulky head groups and multiple polar groups causes lateral cohesion to be reduced and films to expand. The S–A curves are quite steeply curved but extrapolation to a limiting surface area yields a value that is usually several times greater than the cross-sectional area from molecular models.
Condensed films: If the concentration of the surfactant is high, it forms a rigid film between the immiscible phases and acts as a mechanical barrier to both adhesion and coalescence of the liquid droplets. The molecules of the long straight-chain fatty acids, such as palmitic acids, are more tightly packed due to the cohesive contact of hydrocarbon chains. As the chains interlock, the molecules do not freely move in the interface, leading to a stable emulsion. In these films, the film pressure remains very low at high film areas and increases abruptly when the molecules become tightly packed on compression.
Interfacial Complex Condensed Films: To improve stability, the combinations of surfactants are often used rather than a single surfactant. Combination of a water-soluble surfactant that produces a gaseous film and an oil-soluble auxiliary surfactant produces a stable interfacial complex condensed film. This film is flexible, highly viscous, coherent, elastic and resistant to rupture since the molecules are efficiently packed between each other. Thus, a tightly packed surfactant film explains the well-known fact that mixed surfactants are often more effective than single surfactants. The ability of the mixture of surfactants to pack more tightly contributes to the strength of the film, and hence, to the enhanced stability.
Lamellar Liquid Crystalline Films: Stable emulsions are believed to comprise liquid crystalline layers on the interface of emulsified droplets with the continuous phase. Mixed emulsifiers can interact with water to form three- dimensional association structures. Emulsions should be viewed as three-component systems comprising oil, water and lamellar liquid crystals, the latter consisting of consecutive layers of water–emulsifier–oil–water.
Multiple choice questions (MCQs)
1.Surface-active agents tend to concentrate at interfaces and are adsorbed at oil–water interfaces as monomolecular films. These monomolecular films formed at the interface depend on which of the following?
a)Nature
b)Characteristics
c)Concentration and combination of the surfactant
d)All of these
2.The properties of the film can be studied using
a)Film balance
b)Viscometer
c)Stalagmometer
d)All of these
3.Which of the films are expanded films?
a)Films formed by oleic acid
b)Film formed by dissolving surfactant in a volatile solvent
c)Film formed if the concentration of the surfactant is high
d)All of these
4.Which of the following is condensed film?
a)Films formed by oleic acid
b)Film formed by dissolving surfactant in a volatile solvent
c)Film formed if the concentration of the surfactant is high
d)All of these
5.Which of the following is gaseous film?
a)Films formed by oleic acid
b)Film formed by dissolving surfactant in a volatile solvent
c)Film formed if the concentration of the surfactant is high
d)If adsorbed surfactant molecules do not adhere to each other laterally
6.Why Interfacial Complex Condensed Films are formed?
a)To improve stability
b)To increase solubility
c)To increase viscosity
d)All of these
7.The ability of the mixture of surfactants to pack more tightly contributes to the
a)Strength of the film
b)Enhanced stability
c)Both of these
d)None of these
8.What is the main result of adding surfactants into a liquid composed of two immiscible phases such as oil and water?
a)Reduction in the interfacial tension between the phases
b)Increase in the interfacial tension between the phases
c)Catalysation of a chemical reaction between the phases
d)Nothing happens
9.Insoluble amphiphilic compounds such as surfactants with very long hydrocarbon chains form films one molecule in thickness on water surfaces when the surfactant is dissolved in a volatile solvent and carefully injected onto the surface. This type of layer formed is called
a)Insoluble Monomolecular Films
b)Gaseous film
c)Expanded films
d)Condensed films
10.S–A curves are plotted in which type of films?
a)Insoluble Monomolecular Films
b)Gaseous film
c)Expanded films
d)Condensed films
11.Which of the following is not an adsorbent?
a) Carbon
b) Polymers and resins
c) Clay
d) Dry sponge
12.What do you mean by the term “Sorption”?
a)Attachment
b)Detachment
c)Diffusion
d)Thermal Expansion
13.One example of a gaseous film is
a)Film that is formed by the anionic surfactant, sodium dodecyl sulphate
b)Water–emulsifier–oil–water film
c) Both of these
d)None of these
14.Which of the following statements regarding the physical adsorption of a gas on surface of solid is not correct?
a)On increasing temperature, adsorption increases continuously
b)Enthalpy changes are negative
c)Adsorption is specific
d)It is reversible in nature
15.Which of the following is not characteristic of chemisorption?
a)It is irreversible
b)It is specific
c)It is multilayer phenomenon
d)Heat of adsorption is about 400kj
Solutions:
- d)All of these
- a)Film balance
- a)Films formed by oleic acid
- c)Film formed if the concentration of the surfactant is high
- d)If adsorbed surfactant molecules do not adhere to each other laterally
- a)To improve stability
- c)Both of these
- a) Reduction in the interfacial tension between the phases
- a)Insoluble Monomolecular Films
- a)Insoluble Monomolecular Films
- d) Dry sponge
- a) Attachment
- a)Film that is formed by the anionic surfactant, sodium dodecyl sulphate
- a) On increasing temperature, adsorption increases continuously
- c) It is multilayer phenomenon
References:
1. GAURAV KUMAR JAIN – THEORY & PRACTICE OF PHYSICAL PHARMACY, 1st edition 2012 Elsevier, page no. 127-128.
2. Martins Physical Pharmacy, 6th edition 2011, page no. 681-698.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
The post Surface and interfacial phenomenon: Complex films and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>The post Surface and interfacial phenomenon: Solubilization and detergency and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>SOLUBILITY: The maximum amount of solute that can be dissolve in a given amount of solvent.
FACTORS THAT EFFECT SOLUBILIZATION:
NATURE OF SOLUTE AND SOLVENT: ◦ The amount of solute that dissolves depends on what type of solute it is. While only 1 gram of lead (II) chloride can be dissolved in 100 grams of water at room temperature, 200 grams of zinc chloride can be dissolved. This means that a greater amount of zinc chloride can be dissolved in the same amount of water than lead II chloride.
TEMPERATURE: Generally in many cases solubility increases with the rise in temperature and decreases with the fall of temperature but it is not necessary in all cases. However we must follow two behaviors:
In endothermic process solubility increases with the increase in temperature and vice versa EXAMPLE: solubility of potassium nitrate increases with the increase in temperature.
In exothermic process solubility decrease with the increase in temperature. EXAMPLE: solubility of calcium oxide decreases with the increase in temperature.
Gases are more soluble in cold solvent than in hot solvent.
PRESSURE: For solid and liquid solutes, changes in pressure have practically no effect on solubility For gaseous solutes, an increase in pressure increases solubility and a decrease in pressure decreases solubility.
PARTICLE SIZE: Solubility will increase with the decrease size of solute particle because of the additional surface energy. This effect is generally small unless particles become very small typically smaller than 1 micro meter.
Detergency: It is most important property of surface active agents. Surface active agents are referred as detergents. The term Detergency is mostly used in the cleaning / removing of grease, oil and dirt from the solid surface. The principle of detergency is based on the formation of micelle.
The process needs many of the actions specific to surfactant molecules.
- The surfactant requires good wetting properties to ensure good contact with the solid surface.
- It also has the ability to remove dirt into the bulk liquid.
- This property is achieved by lowering the surface tension of the medium in which surfactants is dissolved.
- By lowering this interfacial tension between two media or interfaces (e.g. air/water, water/stain, stain/fabric) the surfactant plays a key role in the removal and suspension of dirt.
- The lower surface tension of the water makes it easier to lift dirt and grease off of dirty dishes, clothes and other surfaces, and help to keep them suspended in the dirty water.
- The water-loving or hydrophilic head remains in the water and it pulls the stains towards the water, away from the fabric.
- The surfactant molecules surround the stain particles, break them up and force them away from the surface of the fabric.
- They then suspend the stain particles in the wash water to remove them. If the dirt is oily it may be emulsified or solubilized by the surfactant.
MICELLES: When surfactant are added to water, they self-assemble into little sphere called micelles with the hydrophilic head facing out and hydrophobic tail pointing in.
TYPES OF MICELLES:
SPHERICAL MICELLES: The micelles which are arranged in spherical form are called spherical micelles.
ROD SHAPE MICELLES: The micelles which are arranged in rod form are called rod shaped micelles.
LAMELLAR MICELLES: The micelles which are arranged in plates (lamellae) form are called lamellar micelles.
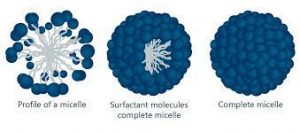
Fig 1 – Micelle (taken from Micelle Kruss scientific)
FORMATION OF MICELLE:
Micelles form when the polar head and the non polar tails arrange in a special way. They are usually driven to arrange either with the polar heads out (oil in water) or with the polar head in (water in oil). Micelles only form when the concentration of surfactant is greater than the critical micelle concentration (CMC). The surfactant is any surface active material that can part the surface upon entering. The CMC is the concentration above surfactant when micelles will form spontaneously. The higher the concentration, the more micelles there are. Micelle formation also depend on the Krafft temperature. This temperature is when surfactants will form micelles. If the temperature is below the Krafft temperature, then there is no spontaneous formation of micelles. As the temperature increases, the surfactant will turn into a soluble form and be able to form micelles from a crystalline state. The hydrophobic effect is also a driving force that needs to be taken into account. This effect is characterized by the fact that like to form intermolecular aggregates in aqueous substances and in intramolecular molecules. Micelle formation can be summed up by thermodynamics, driven by entropy and enthalpy.
Multiple choice questions (MCQs)
1.Which of the following process does not occur at the interface of phases?
a)Crystallization
b)Heterogenous catalysis
c)Homogeneous catalysis
d)Corrosion
2.The preparation of a thermodynamically stable isotropic solution of a substance normally insoluble or very slightly soluble in a given solvent by the addition of component or by any suitable methods is called
a)Solubilization
b)Solubility
c)Both of these
d)None of these
3.The maximum amount of solute that can be dissolve in a given amount of solvent is known as
a)Solubilization
b)Solubility
c)Both of these
d)None of these
4.Which of the following factors effect solubilization?
a)Temperature
b)Pressure
c)Particle size
d)All of the above
5.Generally in many cases solubility _________ with the rise in temperature
a)Increases
b)Decreases
c)No change
d)There is no relation between temperature and solubilization
6.In endothermic process solubility _____ with the increase in temperature
a)Increases
b)Decreases
c)No change
d)There is no relation between temperature and solubilization
7.In exothermic process solubility _____ with the increase in temperature
a)Increases
b)Decreases
c)No change
d)There is no relation between temperature and solubilization
8.For gaseous solutes, an increase in pressure _____ solubility
a)Decrease
b)Increase
c)No change
d)There is no relation between pressure and solubilization
9.Solubility will ____ with the decrease size of solute particle
a)Decrease
b)Increase
c)No change
d)There is no relation between particle size and solubilization
10.Surface active agents are referred as
a)Hydrocolloids
b)Buffering agents
c)Detergents
d)all of the above
11.At high concentration of soap in water, soap behaves as ____________.
a)Molecular colloid
b)Associated colloid
c)Macromolecular colloid
d)Lyophilic colloid
12.When surfactant are added to water, they self-assemble into little sphere called
a)Hydrocolloids
b)Floccules
c)Micelles
d)All of the above
13.Which of the following are types of micelle?
a)Spherical
b)Rod shaped
c)Lamellar
d)All of the above
14.Micelles only form when the concentration of surfactant is greater than the
a)CMC
b)Oil globules
c)Water molecules
d)Anions
15.Which of the following options are correct?
a)Micelle formation by soap in aqueous solution is possible at all temperatures.
b)Micelle formation by soap in aqueous solution occurs above a particular concentration.
c)Soap solution behaves as a normal strong electrolyte at all concentrations.
d)None of above
Solutions:
- c) homogeneous catalysis
- a)Solubilization
- b)Solubility
- d)All of the above
- a) increases
- a)increases
- b)decreases
- b)increase
- b)increase
- c)detergents
- b) associated colloid
- c)micelles
- d)All of the above
- a)CMC
- b) Micelle formation by soap in aqueous solution occurs above a particular concentration.
References:
- Martins Physical Pharmacy, 6th edition 2011, page no. 670-680.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
The post Surface and interfacial phenomenon: Solubilization and detergency and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>The post Hydrophilic–Lipophilic Balance Concept, HLB classification and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>- The higher the HLB number, the more hydrophilic is the surfactant.
- The lower the HLB number, the more lipophilic is the surfactant.
- Exceptions to HLB scale exist such as sodium lauryl sulphate with an HLB value of 40.
Table 1 – Application of surfactants based on HLB value
HLB values Surfactants
15–18 Solubilizing agents (sodium lauryl sulphate)
13–15 Detergents (sodium stearate)
8–16 O/W emulsifer (Tween)
7–9 Wetting agents (Acacia)
3–6 W/O emulsi¿er (Span)
1–3 Antifoaming agents (simethicone)
Calculation of HLB value:
- HLB values of surfactants based on polyhydric alcohol fatty acid esters such as glyceryl monostearate, sorbitan monooleate and polyoxyethylene sorbitan monooleate may be estimated by the following equation:
HLB = 20(1-S/A)
where S is the saponification number of the ester and A is the acid number of the fatty acid.
- For materials such as beeswax and lanolin derivatives with which it is not possible to obtain good saponification number, the HLB value is estimated by the following:
HLB = E + P/5
where E is the weight percentage of oxyethylene chain and P is the weight percentage
of polyhydric alcohol groups (e.g. glycerol or sorbitol) in the material.
- For materials whose hydrophilic region is polyoxyethylene, the HLB value is calculated by the following:
HLB = E/5
- In another method for calculating the HLB values, the component groups of the surfactant molecules are assigned group numbers and these are then added to give the HLB value of the surfactant molecule.
HLB = Ɛ (hydrophilic group numbers) − Ɛ (lipophilic group numbers) + 7
Required HLB- Generally, a single emulsifier cannot yield the desired type of emulsion. More often, stable emulsions can be prepared by utilizing a combination of a hydrophilic and a lipophilic surfactant. Such combinations produce mixed interfacial phases of high surface coverage as well as of sufficient viscosity to prevent creaming.
HLB values of combinations of surfactants A (HLBA) and B (HLBB) are calculated by the following equation:
HLBmixture = fA X HLBA + (1-fA) X HLBB
Multiple choice questions (MCQs)
1.The HLB concept was introduced by
a)Sorensen
b)Griffin
c)Arhenius
d)Seydler
2.The HLB concept was introduced in
a)1947
b)1950
c)1951
d)1955
3.The higher the HLB number, the more _____ is the surfactant
a)Hydrophilic
b)Lipophilic
c)Amphiphilic
d)All of the above
4.The lower the HLB number, the more _____ is the surfactant.
a)Hydrophilic
b)Lipophilic
c)Amphiphilic
d)All of the above
5.Sodium lauryl sulphate has an HLB value of
a)3
b)12
c)18
d)40
6.Solubilizing agents have HLB value
a)15–18
b)8–16
c)7–9
d)3–6
7.Detergents have HLB value
a)15–18
b)8–16
c)7–9
d)13–15
8.O/W emulsifer have HLB value
a)15–18
b)8–16
c)7–9
d)13–15
9.Wetting agents have HLB value
a)15–18
b)8–16
c)7–9
d)13–15
10.W/O emulsifier have HLB value
a)8–16
b)7–9
c)13–15
d)3-6
11.Antifoaming agents have HLB value
a)8–16
b)7–9
c)13–15
d)1-3
12.HLB values of surfactants based on polyhydric alcohol fatty acid esters such as glyceryl monostearate, sorbitan monooleate and polyoxyethylene sorbitan monooleate may be estimated by which of the following equation?
a)HLB = 20(1-S/A)
b)HLB = E + P/5
c)HLB = Ɛ (hydrophilic group numbers) − Ɛ (lipophilic group numbers) + 7
d)All of the above
13.For materials such as beeswax and lanolin derivatives with which it is not possible to obtain good saponification number, the HLB value is estimated by which of the following?
a)HLB = 20(1-S/A)
b)HLB = E + P/5
c)HLB = Ɛ (hydrophilic group numbers) − Ɛ (lipophilic group numbers) + 7
d)All of the above
14.For materials whose hydrophilic region is polyoxyethylene, the HLB value is calculated by which of the following?
a)HLB = 20(1-S/A)
b)HLB = E + P/5
c)HLB = Ɛ (hydrophilic group numbers) − Ɛ (lipophilic group numbers) + 7
d)All of the above
15.HLB values of combinations of surfactants A (HLBA) and B (HLBB) are calculated by which of the following equation?
a)HLB = 20(1-S/A)
b)HLB = E + P/5
c)HLB = Ɛ (hydrophilic group numbers) − Ɛ (lipophilic group numbers) + 7
d)HLBmixture = fA X HLBA + (1-fA) X HLBB
Solutions:
- b)Griffin
- a)1947
- a)Hydrophilic
- b)Lipophilic
- d)40
- a)15–18
- d)13–15
- b)8–16
- c)7–9
- d)3-6
- d)1-3
- a)HLB = 20(1-S/A)
- b)HLB = E + P/5
- c)HLB = Ɛ (hydrophilic group numbers) − Ɛ (lipophilic group numbers) + 7
- d)HLBmixture = fA X HLBA + (1-fA) X HLBB
References:
1. GAURAV KUMAR JAIN – THEORY & PRACTICE OF PHYSICAL PHARMACY, 1st edition 2012 Elsevier, page no. 104-107.
2. Martins Physical Pharmacy, 6th edition 2011, page no. 671-676.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
The post Hydrophilic–Lipophilic Balance Concept, HLB classification and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>The post Surfactants, Surface active agents and Question Answer for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>Substances having both hydrophilic and hydrophobic regions in their molecular structures are called surfactants or surface-active agents. When surfactants are added to the air/ liquid (water) interface, they accumulate at the interface, a process that is generally described as adsorption. At the interface, the surfactants orient themselves in a monomolecular layer with the hydrophilic head (polar) pointing towards the water and the hydrocarbon chain (nonpolar) pointing towards the air. Such an orientation expands the interface and lowers the surface tension. If the interfacial tension is decreased sufficiently, the dispersed system will readily be wetted owing to the decrease in contact angle. With the increase in the concentration of the surfactant in an aqueous solution, the interfacial tension is appreciably lowered. Further addition leads to saturation at the surface, where the surfactant molecules are closely packed. Beyond saturation, the excess surfactant moves into the bulk and forms micelles within the aqueous solution, thereby concluding the change in surface tension. The concentration at which micelle formation occurs is termed critical micelle concentration (CMC). In the micelle, the surfactant hydrophobic groups are directed towards the interior of the aggregate and the polar head groups are directed towards the solvent; thus, micelles help in solubilization of the dispersed phase.
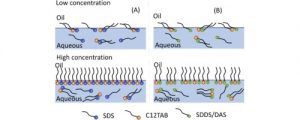
Fig 1 – Arrangement and orientation of surfactant molecules at surface (at low concentration) and in bulk solution (at high concentration) (taken from science direct)
Classification of Surfactants:
- Anionic surfactants
- Cationic surfactants
- Ampholytic surfactants
- Nonionic surfactants
- Polymeric surfactants
Anionic surfactants- Anionic surfactants in common use consist of the soaps of alkali, amines and metals, sulphated alcohols and sulphonates. On dissociation, the long-chain anion (negative charge) of these surfactants imparts surface activity, whereas the cation is inactive. These agents are however not suitable for internal use because of their unpleasant taste and irritant action on the intestinal mucosa.
Table 1 – Types of anionic surfactants
| Types | Description |
| Alkali soaps (sodium and potassium stearate) | 1. Sodium, potassium and ammonium salts of long-chain fatty acids (stearic and oleic acid)
2. Unstable below pH 10 and are incompatible with acids and polyvalent inorganic and long-chain organic cation.s |
| Metallic soaps (calcium stearate) | Salts of divalent and trivalent metals (calcium, magnesium, zinc and aluminium) with long-chain fatty acids. |
| Amine soaps | Formed by reaction between amines (ethanolamine, diethanolamine and triethanolamine) and fatty acids (oleic acid). |
| Alkyl sulphates and phosphates (sodium lauryl sulphate) | Esters formed by reaction of fatty alcohols with sulphuric acid and phosphoric acid. |
| Alkyl sulphonates (sodium dioctyl sulphosuccinate also known as aerosol AT) | Effective wetting agent. |
Cationic surfactants- In aqueous solutions, cationic surfactants dissociate to form positively charged cations, which give them emulsifying properties. Quaternary ammonium compounds such as cetyltrimethylammonium bromide (cetrimide), benzethonium chloride and benzalkonium chloride are examples of important cationic surfactants.
- More popular as antiseptics or disinfecting agents due to their bactericidal action.
- Widely used as preservatives and for sterilizing contaminated surfaces.
- Secondary emulsifying agents for external application.
- Incompatible with anionic surfactants and are unstable at high pH.
Ampholytic surfactants- Ampholytic surfactants possess both cationic and anionic groups in the same molecule and their ionic characteristics depend on the pH of the system. Below a certain pH value they behave as cations, above a certain pH value they behave as anions, and at intermediate pH, they behave as zwitterions. Examples of ampholytic surfactants include lecithin and N-dodecyl alanine.
- Lecithin is used as emulsifier for parenteral applications.
Nonionic surfactants- Unlike anionic and cationic surfactants, nonionic surfactants are useful for oral and parenteral formulations because of their low irritation and toxicity. Based on their neutral nature, they are much less sensitive to changes in the pH of the medium and the presence of electrolytes. These are available in various hydrophile–lipophile balances (HLBs), which stabilize oil-in-water (O/W) or water-in-oil (W/O) emulsions.
Table 2 – Types of nonionic surfactants
| Types | Description |
| Sorbitan esters (Spans) | 1. Products of the esterification of a sorbitan with a fatty acid
2. Low HLB number, insoluble in water and used as W/O emulsifiers. |
| Polysorbates (Tweens) | 1. Ethoxylated derivatives of sorbitan esters
2. High HLB number, soluble in water and used as O/W emulsifiers. |
Polymeric surfactants- The most commonly used polymeric surfactants used in pharmacy are the A–B–A block copolymers, with A being the hydrophilic chain [poly(ethylene oxide), PEO] and B being the hydrophobic chain [poly(propylene oxide), PPO]. The general structure is PEO–PPO–PEO and is commercially available with different proportions of PEO and PPO (Pluronics,Synperonic and Poloxamers). Another important class of polymeric surfactants that are used for demulsification is those based on alkoxylated alkylphenol formaldehyde condensates. Silicone surfactants with a poly(dimethyl siloxane) backbone can cause enhanced wetting and spreading of their aqueous solution.
Used to prepare highly stable concentrated suspensions.
Multiple choice questions (MCQs)
1.Substances having both hydrophilic and hydrophobic regions in their molecular structures are called
a)Surface active agents
b)Surfactants
c)Both of these
d)None of these
2.When surfactants are added to the air/ liquid (water) interface, they accumulate at the interface, a process that is generally described as
a)Absorption
b)Adsorption
c)Capillary action
d)All of these
3.The concentration at which micelle formation occurs is termed
a)Kraft point
b)Cloud point
c)isoelectric point
d)CMC
4.Which of the following are types of surfactants?
a)Anionic surfactants
b)Cationic surfactants
c)Nonionic surfactants
d)All of the above
5.Which of the following are anionic surfactants?
a)Soaps of alkali
b)Sulphated alcohols
c)Amines and metals
d)All of the above
6.Which of the following is/are cationic surfactant?
a)Benzethonium chloride
b)N-dodecyl alanine
c)Salts of divalent and trivalent metals
d)All of the above
7.Lecithin is used as emulsifier for
a)Opthalmics
b)Parenterals
c)Both of these
d)Nne of these
8.Which of the following are ampholytic surfactants?
a)N-dodecyl alanine
b) Lecithin
c)Both of these
d)None of these
9.Which of the following is/are nonionic surfactants?
a)Ethanolamine
b)Sorbitan esters
c)Stearic and oleic acid
d)All of the above
10.Which of the following is used to prepare highly stable concentrated suspensions?
a)Anionic surfactants
b)Cationic surfactants
c)Ampholytic surfactants
d)Polymeric surfactants
11.The most commonly used polymeric surfactants used in pharmacy are
a)A–B–A block copolymers
b)B_A_B block copolymers
c)A_A_A block copolymers
d)B_B_B block copolymers
12.Which of the following is correct statement regarding sorbitan esters?
a)Products of the esterification of a sorbitan with a fatty acid
b)Low HLB number, insoluble in water and used as W/O emulsifiers
c)Both of these
d)None of these
13.Which of the following is correct statement regarding polysorbates?
a)Ethoxylated derivatives of sorbitan esters
b)High HLB number, soluble in water and used as O/W emulsifiers
c)Both of these
d)None of these
14.Sorbitan esters are
a)Spans
b)Tweens
c)Both of these
d)None of these
15.Polysorbates are
a)Spans
b)Tweens
c)Both of these
d)None of these
Solutions:
- c)Both of these
- b)Adsorption
- d)CMC
- d)All of the above
- d)All of the above
- a)Benzethonium chloride
- b)Parenterals
- c)Both of these
- b)Sorbitan esters
- d)Polymeric surfactants
- a)A–B–A block copolymers
- c)Both of these
- c)Both of these
- a)Spans
- b)Tweens
References:
1. GAURAV KUMAR JAIN – THEORY & PRACTICE OF PHYSICAL PHARMACY, 1st edition 2012 Elsevier, page no. 99-103.
2. Martins Physical pharmacy, 6th edition 2011, page no. 670-675.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
The post Surfactants, Surface active agents and Question Answer for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>The post Langmuir isotherm and Freundlich isotherm, Adsorption Phenomenon (Part2) and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>Langmuir isotherm: Langmuir tried to explain adsorption in terms of dynamic equilibrium between the rates of adsorption and desorption. He derived an equation based on the following facts:
- Every active site of the adsorbent acts in the same way
- Adsorbate is adsorbed on the surface of the solid (adsorbent) to form a monolayer
- The rate of adsorption is proportional to the concentration of the adsorbate [A] and the number of unoccupied sites available (1 – α)
Rate of adsorption of solute = ka [A] (1 – α)
where ka is the adsorption rate constant.
- The adsorbed substances tend to escape from the surface and therefore the rate of desorption is proportional to the number of occupied sites (α)
Rate of desorption of solute = kd α
where kd is the desorption rate constant.
- At equilibrium, the rates of adsorption and desorption are equal
ka [A] (1 – α) = kd α
or
α = ka [A] / ka [A] + kd
If a monolayer of the solute covers the surface of the adsorbent, the amount of solute, adsorbed per unit weight of adsorbent, is directly proportional to the fraction D of the surface occupied with the solute:
qe = k’ α
where k’ is a constant. Substituting above equations and dividing the resulting equation by kd yields the following:
qe = (ka k’/ kd) [A] / 1 + (ka/kd) [A] = k1A/1+k2A
where, k1 = ka k’/ kd and k2 = ka/kd
In a double reciprocal form, the equation is rearranged as follows:
1/ qe = k1/ k2 + 1/ k1 (1/[A])
Hence the plot of 1/qe versus 1/[A] gives a slope of 1/K1 and an intercept of K1/K2.
Freundlich isotherm: There are two special cases of the Langmuir isotherm.
For very low concentrations (i.e. K2[A] << 1) the specific adsorption is proportional to the concentration of the adsorbate:
qe = k1/[A]
For very high concentrations (i.e. K2[A] >> 1) the specific adsorption is independent of the concentration of the adsorbate:
qe = k1/k2
For intermediate concentration, the Freundlich equation for adsorption at a given temperature is
qe = k[A]n
where k and n are constants and the value of n ranges from 0 to 1.
When n = 1, the Freundlich equation is identical to the very low concentration case of the Langmuir isotherm.
When n = 0, the Freundlich equation is identical to the very high concentration case of the Langmuir isotherm.
The logarithmic form of Freundlich equation is
log qe = log k + n log [A]
Plotting qe against log [A] gives a straight line with a slope n and intercept log k
Multiple choice questions (MCQs)
1.BET stands for
a)Brunner, Emmett and Teller
b)Burner, Emmett and Teller
c)Brunner, Emet and Teller
d)Brunner, Emmett and Tailor
2.Langmuir tried to explain adsorption in terms of _____ between the rates of adsorption and desorption.
a)Dynamic equilibrium
b)Static equilibrium
c)Both of these
d)None of these
3.Langmuir derived an equation based on which of the following facts?
a)Every active site of the adsorbent acts in the same way
b)Adsorbate is adsorbed on the surface of the solid (adsorbent) to form a monolayer
c)The rate of adsorption is proportional to the concentration of the adsorbate [A] and the number of unoccupied sites available (1 – α)
d)All of these
4.In adsorption at liquid interface, when the added molecules move on their own according to the interface, this process is called
a)Negative adsorption
b)Positive absorption
c)Positive desorption
d)Positive adsorption
5.The Freundlich equation is identical to the very low concentration case of the Langmuir isotherm when
a)n = 1
b)n = 0
c)n>1
d)n=2
6.The Freundlich equation is identical to the very high concentration case of the Langmuir isotherm when
a)n = 1
b)n = 0
c)n>1
d)n=2
7.According to Freundlich adsorption isotherm, which of the following is correct?
a)x/m α p1/n
b)x/m α p1
c)x/m α pº
d)all are correct at different ranges of pressure
8.According to Langmuir isotherm the adsorbed substances tend to escape from the surface and therefore the rate of desorption is proportional to the number of occupied sites (α).
a)True
b)False
9.A plot of log x/m versus log p for the adsorption of a gas on a solid gives a straight line with slope equal to
a)N
b)1/n
c)Log k
d)–log k
10.According to Langmuir isotherm at equilibrium, the rates of adsorption and desorption are equal.
a)True
b)False
11.For very high concentrations (i.e. K2[A] << 1) the specific adsorption is proportional to the concentration of the adsorbate.
a)True
b)False
12.For very high concentrations (i.e. K2[A] >> 1) the specific adsorption is ____ of the concentration of the adsorbate.
a)Dependent
b)Independent
c)More
d)Less
13.For very low concentrations (i.e. K2[A] << 1) the specific adsorption is _____ to the concentration of the adsorbate.
a)Proportional
b)Inversely proportional
c)Independent
d)Higher
14.For intermediate concentration, the Freundlich equation for adsorption at a given temperature is
a)qe = k1/[A]
b)qe = k[A]n
c)qe = k1/k2
d)log qe = log k + n log [A]
15.The logarithmic form of Freundlich equation is
a)qe = k1/[A]
b)qe = k[A]n
c)qe = k1/k2
d)log qe = log k + n log [A]
Solutions:
- a)Brunner, Emmett and Teller
- a)Dynamic equilibrium
- d)All of these
- d)Positive adsorption
- a)n = 1
- b)n = 0
- d)all are correct at different ranges of pressure
- a)True
- b)1/n
- a)True
- b)False
- b)Independent
- a)Proportional
- b)qe = k[A]n
- d)log qe = log k + n log [A]
References:
1. GAURAV KUMAR JAIN – THEORY & PRACTICE OF PHYSICAL PHARMACY, 1st edition 2012 Elsevier, page no. 128-136.
2. Martins Physical Pharmacy, 6th edition 2011, page no. 669-696.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
The post Langmuir isotherm and Freundlich isotherm, Adsorption Phenomenon (Part2) and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>The post BET isotherm, Factors Affecting Adsorption, Adsorption Phenomenon(Part 3) and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>where [A]s is the saturated concentration of the adsorbate, qo is the number of moles of the adsorbate adsorbed per unit weight of adsorbent in a monolayer and b is a constant related to the energy of interaction with the surface.
Plotting the left hand-side term of the equation versus [A]/[As] gives a slope of (b – 1)/bqo and an intercept of 1/bq0. For a simple monomolecular layer, the BET equation reduces to the Langmuir equation.
Factors Affecting Adsorption:
The adsorption of solute molecules from its solution may be influenced by the following factors:
- Nature of adsorbent: The physicochemical nature of the adsorbent can have decisive impacts on the rate and capacity for adsorption. Every solid material can be used as an adsorbent, but activated carbon and clays such as kaolin and bentonite have been used as particular adsorbents in pharmaceutical applications.
- Nature of adsorbate: The solubility of the adsorbate in the solvent from which adsorption takes place has an inverse relationship with the extent of adsorption (Lundelius’ rule). The forces between the adsorbate and solvent need to be broken for adsorption to occur. Thus, higher the solubility of the adsorbate in a solvent, the greater the forces and the smaller the extent of adsorption.
- Adsorbent–solute interaction: Adsorption of a solute from a dilute solution involves the breaking of bonds between the solute and the solvent molecules as well as the formation of bonds between the solute and adsorbent molecules. As an example, the higher molecular weight solutes are usually more readily adsorbed than low molecular weight solutes. This is due to van der Waals forces of attraction, which increases with the size of molecules.
- Adsorbate concentration: The amount of adsorption increases with the increase in the concentration of solute at equilibrium until it reaches a limiting value. However, the relative amount of solute removed from the solution is greater in dilute solutions.
- Surface area of adsorbent: Adsorption is a surface phenomenon and the amount of solute adsorbed depends on the surface area available. Thus, reducing the particle size of the adsorbent will increase the adsorption.
- Temperature: Physical adsorption is an exothermic process and thus a decrease in temperature will increase the extent of adsorption.
- Removal of adsorbed impurities: Removal of adsorbed impurities such as gases or moisture from the surface of solid adsorbent activates the active adsorption sites and increases the efficiency of adsorbents. This can be achieved by heating the adsorbent at high temperature(at 110°C for 1 h).
- pH of the medium: pH of a solution influences the extent of adsorption since pH affects both the degree of ionization and the solubility of the adsorbate drug molecule. More ionized (i.e.polar) and soluble adsorbates adsorb much less than their unionized forms (i.e. lipophilic).Amphoteric adsorbates such as proteins are usually best adsorbed at the isoelectric point where the net charge of the adsorbate becomes zero, and at the lowest solubility.
Multiple choice questions (MCQs)
1.Which of the following factors effect adsorption?
a)Nature of adsorbent
b)pH of medium
c)Temperature
d)All of the above
2.Which of the following are used as particular adsorbents in pharmaceutical applications?
a)Activated carbon
b)Clays
c)Kaolin and Bentonite
d)All of the above
3.The solubility of the adsorbate in the solvent from which adsorption takes place has a/an _____ relationship with the extent of adsorption
a)Direct
b)Inverse
c)No relation
d)Stable
4.The higher molecular weight solutes are usually more readily adsorbed than low molecular weight solutes.
a)True
b)False
5.The amount of adsorption _____ with the increase in the concentration of solute at equilibrium until it reaches a limiting value.
a)Increase
b)Decreases
c)No change
d)Both a nd b
6.The solubility of the adsorbate in the solvent from which adsorption takes place has an inverse relationship with the extent of adsorption. This rule is known as
a)Schulze hardy rule
b)Lundelius’ rule
c)Hofmeister rule
d)Avgadro’s rule
7.Adsorption is a
a)Bulk phenomena
b)Surface phenomena
c)Absorption phenomena
d)All of these
8.Reducing the particle size of the adsorbent will ____ the adsorption.
a)Increases
b)Is infinite
c)Remains unchanged
d)May decrease or increase depending upon size
9.Physical adsorption is an
a)Endothermic process
b)Exothermic process
c)Both of these
d)None of these
10.Decrease in temperature will ____ the extent of adsorption.
a)Increase
b)Decreases
c)No change
d)Both a nd b
11.Removal of adsorbed impurities such as gases or moisture from the surface of solid adsorbent activates the active adsorption sites and increases the efficiency of adsorbents. This can be achieved by heating the adsorbent at
a)110°C for 1 h
b)110°C for 2 hrs
c)110°C for 30 minutes
d)110°C for 20 minutes
12.pH of a solution influences the extent of adsorption since pH affects
a)Degree of ionization
b)Solubility of the adsorbate drug molecule
c) Both of these
d)None of these
13.Which of the following adsorbs lesser?
a)Ionized adsorbates
b)Soluble adsorbates
c)Both of these
d)None of these
14.Amphoteric adsorbates such as proteins are usually best adsorbed at
a)Kraft point
b)CMC
c)Isoelectric point
d)Cloud point
15.At the isoelectric point the net charge of the adsorbate becomes
a)1
b)0
c)2
d)<1
Solutions:
- d)All of the above
- d)All of the above
- b)Inverse
- a)True
- a)Increase
- b)Lundelius’ rule
- b)Surface phenomena
- a)Increases
- b)Exothermic process
- a)Increase
- a)110°C for 1 h
- c) Both of these
- c)Both of these
- c)Isoelectric point
- b)0
References:
1. GAURAV KUMAR JAIN – THEORY & PRACTICE OF PHYSICAL PHARMACY, 1st edition 2012 Elsevier, page no. 128-136.
2. Martins Physical Pharmacy, 6th edition 2011, page no. 669-696.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
The post BET isotherm, Factors Affecting Adsorption, Adsorption Phenomenon(Part 3) and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>The post Physical and chemical adsorption, Adsorption Isotherm, Adsorption phenomenon(Part1) and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>Two different types of adsorption processes exist: physical adsorption (physisorption) and chemical adsorption (chemisorption).
In physical adsorption, adsorbates adsorb on the surface of a solid by van der Waals forces, which are relatively weak and nonspecific forces. The adsorbate is not fixed to the surface of the solid and can move freely within the interfacial surface. Physical adsorption is fast, reversible and results in multilayer adsorption.
In chemical adsorption, the substance is held on the surface of a solid by specific covalent forces between the adsorbate and the adsorbent. The chemically adsorbed materials are not free to move on the surface. Chemical adsorption is slow, not readily reversible and results in monolayer adsorption.
Table 1 – Physical and chemical adsorption
| Parameter | Physical adsorption | Chemical adsorption |
| Force | Weak van der Waals forces | Strong forces |
| Specificity | Non-Specificity | Specificity |
| Reversibility | Reversible | Often irreversible |
| Effect of temperature | Exothermic, adsorption decreases as temperature increases | Surface reaction proceeds above certain temperature |
| Adsorbed layers | Multilayer formation | Monolayer formation |
Adsorption is a dynamic phenomenon that is opposed by desorption, i.e. the transfer of a surfactant to a bulk phase. The adsorption and desorption steps are often very rapid; consequently, adsorption–desorption equilibrium is reached after some time, which depends on the surfactant concentration in the bulk phase. Since the surfactant has a lower free energy when it is adsorbed at the interface than in the solvent bulk phase, the equilibrium is displaced towards the adsorbed state. In fact, the interface is rapidly covered by a monolayer of surfactant and everything happens as if the interface is coated with a thin layer of a new material.
Adsorption Isotherm: When a substance moves away from a solution and adsorbs at the surface of a solid, the concentration of the solute remaining in solution is in dynamic equilibrium with the adsorbed concentration at the surface. This distribution ratio of the solute in solution and at the surface is a measure of the adsorption equilibrium. Adsorption studies using gases generally involve the determination of the amount of gas adsorbed, x, by a given mass, m, of the adsorbent at constant temperature. Determinations are carried out at different equilibrium pressures p(the pressure attained after adsorption) to yield an adsorption isotherm (isotherm refers to a plot at constant temperature). Based on the IUPAC classification, the isotherms obtained can generally be classified into six types as follows:
Table 2 – Adsorption Isotherm
| Type I isotherm: Shows a rapid rise in the adsorption with increasing pressure followed by levelling off.
1. Characteristic of chemical adsorption by microporous adsorbents. 2. Langmuir type isotherms, adsorption being restricted to monolayer. 3. Rapid rise is due to micropore łlling at relatively low pressures. 4. Levelling off is because chemical groups available for chemisorption get saturated very rapidly (monolayer).
|
Example: adsorption of N2 on carbon at 77°K, adsorption of ammonia on charcoal at 273°K. |
| Type II isotherm: Sigmoidal in shape.
1. Characteristic of multilayer physical adsorption onto nonporous solids. 2. First inŃection point is due to the formation of a monolayer. 3. As the pressure is increased further, multilayer formation occurs. 4. Described by BET equation.
|
Example: activated carbons with mixed micro and mesoporosity. |
| Type III isotherm: Convex curve to the relative pressure axis.
1. Characteristic of weak adsorbate–adsorbent interactions and is most associated with nonporous and microporous adsorbents. 2. Low adsorption at low pressures due to weak interactions between the adsorbate and the adsorbent. 3. Accelerated uptakes at higher relative pressure due to strong adsorbate–adsorbate interaction after adsorption at primary sites.
|
Example: adsorption of N2 water on carbon, the primary adsorption sites are oxygen based. |
| Type IV isotherm:
1. Characteristic of adsorption of gases on porous solids. 2. First inŃection point represents the amount of gas required to form a monolayer on the surface of the solid. 3. Further adsorption is due to multilayer formation and capillary condensation within the pores of the solid. 4. Limiting value is due to saturation vapour pressure.
|
Example: condensation of gases on porous solids. |
| Type V isotherm:
1. Similar to Type III isotherm, shows convex curve characteristic of weak adsorbate–adsorbent interactions. 2. Further adsorption is due to capillary condensation as seen for Type IV isotherm. 3. Adsorption reaches a limiting value before the saturation vapour is reached. |
|
| Type VI isotherm: Stepwise isotherm
1. Characteristic of extremely homogeneous, nonporous surfaces where the monolayer capacity corresponds to step height. 2. Formation of complete monolayer before each subsequent multilayer commences. 3. Shows distinct steps corresponding to the complete formation of each monolayer.
|
Example: adsorption of krypton on carbon black at 90K. |
Multiple choice questions (MCQs)
1.The process in which materials of one phase (adsorbate) accumulate or concentrate at the interfacial surface of the other phase (adsorbent) is called
a)Adsorption
b)absorption
c)Both of these
d)None of these
2.Adsorption is a spontaneous phenomenon driven by a reduction of the
a)Surface free energy
b)Bonds between ions
c)Bonds between molecules
d)Density
3.Adsorption occurs at the interfaces of two phases such as
a)liquid/liquid
b)gas/liquid
c)liquid/solid
d)All of the above
4.Which of the following are types of adsorption process?
a)Physical adsorption
b)Physisorption
c)Chemical adsorption
d)All of the above
5.Chemical adsorption is also known as
a)Physisorption
b)Chemisorption
c)Physical adsorption
d)Chemical absorption
6.In physical adsorption, adsorbates adsorb on the surface of a solid by
a)Ionic bonds
b) Van der Waals forces
c)Covalent bonds
d)All of these
7.Physical adsorption is
a)Fast
b)Reversible
c)Multilayer adsorption
d)All of the above
8.In chemical adsorption, the substance is held on the surface of a solid by
a)Ionic bonds
b) Van der Waals forces
c)Covalent bonds
d)All of these
9.Chemical adsorption is
a)Slow
b)Not readily reversible
c)Monolayer adsorption
d)All of the above
10.As per Langmuir adsorption isotherm, the rate of adsorption is proportional to
a)Occupied spots
b)Unoccupied spots
c)Pressure
d)Both a and b
11.Adsorption is a dynamic phenomenon that is opposed by
a)Chemisorption
b)Physisorption
c)Absorption
d)Desorption
12.The term ‘Sorption’ stand for
a)Absorption
b)Adsorption
c)Desorption
d)Both a and b
13.Isotherm refers to a plot at
a)Varying temperature
b)Varying pressure
c)Constant pressure
d)Constant temperature
14.Based on the IUPAC classification, the isotherms obtained can generally be classified into how many types?
a)2
b)4
c)6
d)8
15.Which of the following is example of type II isotherm?
a)Adsorption of N2 on carbon at 77°K, adsorption of ammonia on charcoal at 273°K
b)Activated carbons with mixed micro and mesoporosity
c)Adsorption of krypton on carbon black at 90K
d)Condensation of gases on porous solids
Solutions:
- c)Both of these
- a)Surface free energy
- d)All of the above
- d)All of the above
- b)Chemisorption
- b) Van der Waals forces
- d)All of the above
- c)Covalent bonds
- d)All of the above
- d) Both a and b
- d)Desorption
- d)Both a and b
- d)Constant temperature
- c)6
- b)Activated carbons with mixed micro and mesoporosity
References:
1. GAURAV KUMAR JAIN – THEORY & PRACTICE OF PHYSICAL PHARMACY, 1st edition 2012 Elsevier, page no. 128-136.
2. Martins Physical Pharmacy, 6th edition 2011, page no. 669-696.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
The post Physical and chemical adsorption, Adsorption Isotherm, Adsorption phenomenon(Part1) and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>The post Surface and Interfacial Phenomenon: Spreading coefficient and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>Work of adhesion (Wa)- Consider a liquid drop with surface tension ϒLV and a solid surface with surface tension ϒSV. When the liquid drop adheres to the solid surface, it forms a surface tension ϒSL. The work of adhesion is simply the difference between the surface tensions of the liquid/vapour and solid/vapour and that of the solid/liquid. The work of adhesion is given by the following equation:
Wa = ϒSV + ϒLV – ϒSL
Work of cohesion (Wc)- The work of cohesion is the work of adhesion when the two phases are the same. Consider a liquid cylinder with unit cross-sectional area. When this liquid is subdivided into two cylinders, two new surfaces are formed. The two new areas will have a surface tension of 2ϒLV and the work of cohesion is expressed by the following equation:
Wc = 2ϒLV
The spreading coefficient (S) is the difference between the work of adhesion and the work of cohesion (Wa – Wc). This implies that if the work of adhesion is more than the work of cohesion, spreading will occur.
Then,
Wa – Wc = ϒL + ϒS – ϒLS – 2ϒL
S = ϒL + ϒS – ϒLS
S = ϒS – (ϒL + ϒLS)
where ϒS refers to the surface tension of the sublayer liquid, ϒL refers to the surface tension of spreading liquid and ϒLS refers to the interfacial tension between the two layers.
Spreading occurs when the surface tension of the sublayer liquid is greater than the sum of the surface tension of the spreading liquid and the interfacial tension between the sublayer and the spreading liquid.
- If S is positive or zero, i.e. when ϒS is larger or equal to ϒL + ϒLS, spreading will take place.
- If S is negative, i.e. when ϒL + ϒLS is larger than ϒs, the spreading liquid forms a globule or
a floating lens and spreading will not take place.
- Fatty alcohols and acids have high spreading coefficients because of the presence of polar groups such as OH and COOH, respectively (oleic acid spreads on the surface of water).
- As the nonpolar character of these molecules is increased by increasing the hydrocarbon chain, the spreading coefficient gradually decreases (liquid petroleum fails to spread on water).
- Benzene spreads on water not because of its polar nature but because of its cohesive forces, which are much weaker than the adhesive forces.
Table 1 – Spreading coefficient of some liquids at 20°C
| Liquid | Spreading coefficient (S) (dyne cm -1) |
| Benzene | 8.8 |
| Hexane | 3.4 |
| Octane | 0.2 |
| Toluene | 6.8 |
| Ethanol | 50.4 |
| Acetone | 42.4 |
| Oleic Acid | 24.6 |
| Chloroform | 13.0 |
| Hexadecane | -9.3 |
| Liquid parafłn | -13.4 |
Multiple Choice Questions (MCQs)
1.In general, spreading of a liquid occurs when the work of adhesion between two liquids ____ the work of cohesion between the molecules of each liquid.
a)increases
b)decreases
c)exceeds
d)remains unchanged
2.Spreading Coefficient is denoted by
a)S
b)S.Co
c)Sp
d)Wa
3.Work of adhesion is given by following equation
a)Wa = ϒSV + ϒLV – ϒSL
b)S = ϒL + ϒS – ϒLS
c)Wa – Wc = ϒL + ϒS – ϒLS – 2ϒL
d)Wc = 2ϒLV
4.Work of cohesion (Wc) is given by following equation
a)Wa = ϒSV + ϒLV – ϒSL
b)S = ϒL + ϒS – ϒLS
c)Wa – Wc = ϒL + ϒS – ϒLS – 2ϒL
d)Wc = 2ϒLV
5.If the work of adhesion is more than the work of cohesion, spreading will occur.
a)True
b)False
6.The spreading coefficient (S) is given by following equation
a)Wa – Wc = ϒL + ϒS – ϒLS – 2ϒL
b)S = ϒL + ϒS – ϒLS
c)S = ϒS – (ϒL + ϒLS)
d)All of the above
7.On increasing the temperature, the kinetic energy of the liquid molecules
a)Lowered
b)Remains constant
c)Increases
d)Decreases
8.Spreading occurs when the surface tension of the sublayer liquid is _____ than the sum of the surface tension of the spreading liquid and the interfacial tension between the sublayer and the spreading liquid.
a)smaller
b)greater
c)equal
d)None of the above
9.If S is positive or zero, i.e. when ϒS is larger or equal to ϒL + ϒLS, spreading will take place.
a)True
b)False
10.If S is negative, i.e. when ϒL + ϒLS is larger than ϒs, the spreading liquid forms a globule or
a floating lens and spreading will not take place.
a)True
b)False
11.If adhesive force>cohesive force, then what occurs
a)Wetting
b)Spreading
c)Capillary rise
d)All of above
12.Fatty alcohols and acids have ____ spreading coefficients
a)Lower
b)Higher
c)Optimum
d)No spreading coefficient
13.As the nonpolar character of these molecules is increased by increasing the hydrocarbon chain, the spreading coefficient gradually
a)Increases
b)Decreases
c)Remains unchanged
d)Depends on the nature of hydrocarbon chain
14.The displacement of a solid-air interface with a solid-liquid interface is known as
a)Foaming
b)Antifoaming
c)Wetting
d)Wicking
15.Benzene spreads on water not because of its polar nature but because of its cohesive forces, which are much stronger than the adhesive forces.
a)True
b)False
Solutions:
- c)exceeds
- a)S
- a)Wa = ϒSV + ϒLV – ϒSL
- )Wc = 2ϒLV
- a)True
- d)All of the above
- c)Increases
- b)greater
- a)True
- a)True
- d)All of above
- b)Higher
- b)Decreases
- c)Wetting
- b)False
References:
1. GAURAV KUMAR JAIN – THEORY & PRACTICE OF PHYSICAL PHARMACY, 1st edition 2012 Elsevier, page no. 120-123.
2. Martins Physical Pharmacy, 6th edition 2011, page no. 665-669.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
The post Surface and Interfacial Phenomenon: Spreading coefficient and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>The post Measurement of Surface and Interfacial tension, Ring Detachment Method (du Nouy tensiometer) and Wilhelmy Plate Method and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>The du Nouy’s ring method is similar to the Wilhelmy plate method, but it uses a ring or loop of wire. A platinum or Iridium wire of ~4 cm in circumference is suspended from the loop attached to a scale through a torsion wire. The liquid, whose surface tension is to be determined, is taken in a container. The position of the container is adjusted so that the ring just touches the surface of the liquid. The torsion on the wire is increased gradually so that the ring just detaches from the surface of the liquid.
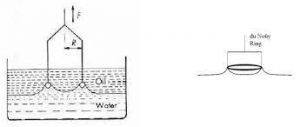
Fig 1 – Du Nuoy tensiometer (taken from standard test method for interfacial tension mastrad test systems)
Again, the detachment force is equal to the surface tension multiplied by the perimeter of liquid detached; hence:
F = W + 4πry
Harkins and Jordan introduced a correction factor f (function of meniscus volume V and radius r of the wire) for more accurate calculation of ᵞ.
F = ᵞ / ᵞideal = (R3/V, R/r)
A correction factor is required before accurate results can be obtained since the above equation does not take into account variables such as the radius of the ring (R), the radius of the wire used to form the ring (r) and the volume of the liquid supported by the ring during detachment (V).
- The ring should lie horizontally in the surface and the wire used to prepare the ring should be absolutely clean.
- The vessel containing the liquid should be large to minimize the influence of the shape of the liquid raised by the ring.
- The surface of the liquid should be free from wave motion.
- Temperature control should be adequate.
Wilhelmy Plate Method:
Principle: The force necessary to detach a plate from the surface or interface of two immiscible liquids is proportional to the surface or interfacial tension, respectively.
The apparatus consists of a thin plate made from glass, mica or platinum attached to a suitable balance. The plate is either detached from the interface (nonequilibrium condition) or its weight is measured statically using an accurate microbalance.
In the detachment method, the plate is immersed in the liquid (whose surface tension is to be measured) taken in a container. The liquid container is then gradually lowered and the reading on the balance immediately prior to detachment is noted. The total force (reading on the balance) F is given by the weight of the plate W and the interfacial tension force.
F = W + ᵞp
where p is the ‘contact length’ of the plate with the liquid, i.e. the plate perimeter. Thus, the detachment force is equal to the surface tension multiplied by the perimeter of the surface detached:
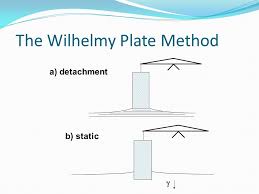
Fig 2 – Wilhelmy plate method (taken from colloid systems myshare.ru)
F – W = 2(L + T)ᵞ
Or
ᵞ = F – W / 2(L + T)
where L and T are the length and thickness of the plate, respectively.
In the static technique, the plate is suspended from one arm of a microbalance and the whole vessel containing the two liquid layers is raised until the interface touches the plate or alternatively the plate is allowed to penetrate the upper liquid layer until it touches the interface. The increase in weight ‘W is given by the following equation:
∆W = ᵞpcosⱷ
Where ⱷ is the contact angle. If the plate is completely wetted by the lower liquid as it penetrates, ⱷ is 0 and ᵞ may be calculated directly from ∆W. The static technique is useful for assessing changes in interfacial tension as a function of time.
- A roughened platinum or glass plate is used to ensure a zero contact angle.
- If the oil is denser than water, a hydrophobic plate is used to ensure its complete wetting.
Multiple choice questions (MCQs)
1.Ring Detachment Method is also known as
a)du Nouy tensiometer
b)Wilhelmy plate method
c)Drop count method
d)Drop weight method
2.Gegenions means
a)Amphiphiles
b)Ions having a charge opposite to the potential determining ions
c)Ions having same charge as that of potential determining ions
d)Potential determining ions
3.A ring or loop of wire used in Ring Detachment Method is made up of
a)Platinum
b)Iridium
c)Both of these
d)None of these
4.What is the circumference of wire used in Ring Detachment Method?
a)2 cm
b)3 cm
c)4 cm
d)5 cm
5.Who introduced a correction factor f (function of meniscus volume V and radius r of the wire) for more accurate calculation of ᵞ?
a)Harkins
b)Jordan
c)Both of these
d)None of these
6.The force necessary to detach a plate from the surface or interface of two immiscible liquids is proportional to the surface or interfacial tension, respectively. This principle is involved in
a)Wilhelmy plate method
b)Drop count method
c)Du Nuoy tensiometer
d)All of the above
7.The apparatus used in Wilhelmy plate method consists of a thin plate made from
a)Glass
b)Mica
c)Platinum
d)All of the above
8.Which of the following are types of Wilhelmy plate method?
a)Detachment method
b)Static method
c)Both of these
d)None of these
9.Which of the following equation is used in Wilhelmy plate method?
a)ᵞ = F – W / 2(L + T)
b)F = W + 4πry
c)∆W = ᵞpcosⱷ
d)F = ᵞ / ᵞideal = (R3/V, R/r)
10.In Ring Detachment Method the contact angle is controlled by
a)Complete wetting
b)Partial wetting
c)Incomplete wetting
d)No wetting
11.Surface tension of water at 25ºC is
a)58dynes/cm
b)68dynes/cm
c)72dynes/cm
d)82dynes/cm
12.Interfacial tension will not exist between
a)Solid-solid
b)Solid-liquid
c)Liquid-liquid
d)Gas-gas
13.ᵞ = F – W / 2(L + T). In this equation L is
a)length of the plate
b)thickness of the plate
c)height of the plate
d)width of the plate
14.ᵞ = F – W / 2(L + T). In this equation T is
a)length of the plate
b)thickness of the plate
c)height of the plate
d)width of the plate
15.In ∆W = ᵞpcosⱷ, ⱷ is
a)contact angle
b)angle of repose
c)both of these
d)none of these
Solutions:
- a)du Nouy tensiometer
- b) Ions having a charge opposite to the potential determining ions
- c)Both of these
- c)4 cm
- c)Both of these
- a)Wilhelmy plate method
- d)All of the above
- c)Both of these
- a)ᵞ = F – W / 2(L + T)
- a)Complete wetting
- c) 72dynes/cm
- d) Gas-gas
- a)length of the plate
- b)thickness of the plate
- a)contact angle
References:
1. GAURAV KUMAR JAIN – THEORY & PRACTICE OF PHYSICAL PHARMACY, 1st edition 2012 Elsevier, page no. 116-118.
2. martins Physical Pharmacy, 6th edition 2011, page no. 662-664.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
The post Measurement of Surface and Interfacial tension, Ring Detachment Method (du Nouy tensiometer) and Wilhelmy Plate Method and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam appeared first on Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts.
]]>