Surfactants, Surface active agents and Question Answer for GPAT, NIPER, Pharmacist and Drug Inspector exam
SURFACE-ACTIVE AGENTS (SURFACTANTS):
Substances having both hydrophilic and hydrophobic regions in their molecular structures are called surfactants or surface-active agents. When surfactants are added to the air/ liquid (water) interface, they accumulate at the interface, a process that is generally described as adsorption. At the interface, the surfactants orient themselves in a monomolecular layer with the hydrophilic head (polar) pointing towards the water and the hydrocarbon chain (nonpolar) pointing towards the air. Such an orientation expands the interface and lowers the surface tension. If the interfacial tension is decreased sufficiently, the dispersed system will readily be wetted owing to the decrease in contact angle. With the increase in the concentration of the surfactant in an aqueous solution, the interfacial tension is appreciably lowered. Further addition leads to saturation at the surface, where the surfactant molecules are closely packed. Beyond saturation, the excess surfactant moves into the bulk and forms micelles within the aqueous solution, thereby concluding the change in surface tension. The concentration at which micelle formation occurs is termed critical micelle concentration (CMC). In the micelle, the surfactant hydrophobic groups are directed towards the interior of the aggregate and the polar head groups are directed towards the solvent; thus, micelles help in solubilization of the dispersed phase.
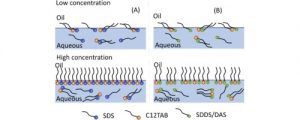
Fig 1 – Arrangement and orientation of surfactant molecules at surface (at low concentration) and in bulk solution (at high concentration) (taken from science direct)
Classification of Surfactants:
- Anionic surfactants
- Cationic surfactants
- Ampholytic surfactants
- Nonionic surfactants
- Polymeric surfactants
Anionic surfactants- Anionic surfactants in common use consist of the soaps of alkali, amines and metals, sulphated alcohols and sulphonates. On dissociation, the long-chain anion (negative charge) of these surfactants imparts surface activity, whereas the cation is inactive. These agents are however not suitable for internal use because of their unpleasant taste and irritant action on the intestinal mucosa.
Table 1 – Types of anionic surfactants
| Types | Description |
| Alkali soaps (sodium and potassium stearate) | 1. Sodium, potassium and ammonium salts of long-chain fatty acids (stearic and oleic acid)
2. Unstable below pH 10 and are incompatible with acids and polyvalent inorganic and long-chain organic cation.s |
| Metallic soaps (calcium stearate) | Salts of divalent and trivalent metals (calcium, magnesium, zinc and aluminium) with long-chain fatty acids. |
| Amine soaps | Formed by reaction between amines (ethanolamine, diethanolamine and triethanolamine) and fatty acids (oleic acid). |
| Alkyl sulphates and phosphates (sodium lauryl sulphate) | Esters formed by reaction of fatty alcohols with sulphuric acid and phosphoric acid. |
| Alkyl sulphonates (sodium dioctyl sulphosuccinate also known as aerosol AT) | Effective wetting agent. |
Cationic surfactants- In aqueous solutions, cationic surfactants dissociate to form positively charged cations, which give them emulsifying properties. Quaternary ammonium compounds such as cetyltrimethylammonium bromide (cetrimide), benzethonium chloride and benzalkonium chloride are examples of important cationic surfactants.
- More popular as antiseptics or disinfecting agents due to their bactericidal action.
- Widely used as preservatives and for sterilizing contaminated surfaces.
- Secondary emulsifying agents for external application.
- Incompatible with anionic surfactants and are unstable at high pH.
Ampholytic surfactants- Ampholytic surfactants possess both cationic and anionic groups in the same molecule and their ionic characteristics depend on the pH of the system. Below a certain pH value they behave as cations, above a certain pH value they behave as anions, and at intermediate pH, they behave as zwitterions. Examples of ampholytic surfactants include lecithin and N-dodecyl alanine.
- Lecithin is used as emulsifier for parenteral applications.
Nonionic surfactants- Unlike anionic and cationic surfactants, nonionic surfactants are useful for oral and parenteral formulations because of their low irritation and toxicity. Based on their neutral nature, they are much less sensitive to changes in the pH of the medium and the presence of electrolytes. These are available in various hydrophile–lipophile balances (HLBs), which stabilize oil-in-water (O/W) or water-in-oil (W/O) emulsions.
Table 2 – Types of nonionic surfactants
| Types | Description |
| Sorbitan esters (Spans) | 1. Products of the esterification of a sorbitan with a fatty acid
2. Low HLB number, insoluble in water and used as W/O emulsifiers. |
| Polysorbates (Tweens) | 1. Ethoxylated derivatives of sorbitan esters
2. High HLB number, soluble in water and used as O/W emulsifiers. |
Polymeric surfactants- The most commonly used polymeric surfactants used in pharmacy are the A–B–A block copolymers, with A being the hydrophilic chain [poly(ethylene oxide), PEO] and B being the hydrophobic chain [poly(propylene oxide), PPO]. The general structure is PEO–PPO–PEO and is commercially available with different proportions of PEO and PPO (Pluronics,Synperonic and Poloxamers). Another important class of polymeric surfactants that are used for demulsification is those based on alkoxylated alkylphenol formaldehyde condensates. Silicone surfactants with a poly(dimethyl siloxane) backbone can cause enhanced wetting and spreading of their aqueous solution.
Used to prepare highly stable concentrated suspensions.
Multiple choice questions (MCQs)
1.Substances having both hydrophilic and hydrophobic regions in their molecular structures are called
a)Surface active agents
b)Surfactants
c)Both of these
d)None of these
2.When surfactants are added to the air/ liquid (water) interface, they accumulate at the interface, a process that is generally described as
a)Absorption
b)Adsorption
c)Capillary action
d)All of these
3.The concentration at which micelle formation occurs is termed
a)Kraft point
b)Cloud point
c)isoelectric point
d)CMC
4.Which of the following are types of surfactants?
a)Anionic surfactants
b)Cationic surfactants
c)Nonionic surfactants
d)All of the above
5.Which of the following are anionic surfactants?
a)Soaps of alkali
b)Sulphated alcohols
c)Amines and metals
d)All of the above
6.Which of the following is/are cationic surfactant?
a)Benzethonium chloride
b)N-dodecyl alanine
c)Salts of divalent and trivalent metals
d)All of the above
7.Lecithin is used as emulsifier for
a)Opthalmics
b)Parenterals
c)Both of these
d)Nne of these
8.Which of the following are ampholytic surfactants?
a)N-dodecyl alanine
b) Lecithin
c)Both of these
d)None of these
9.Which of the following is/are nonionic surfactants?
a)Ethanolamine
b)Sorbitan esters
c)Stearic and oleic acid
d)All of the above
10.Which of the following is used to prepare highly stable concentrated suspensions?
a)Anionic surfactants
b)Cationic surfactants
c)Ampholytic surfactants
d)Polymeric surfactants
11.The most commonly used polymeric surfactants used in pharmacy are
a)A–B–A block copolymers
b)B_A_B block copolymers
c)A_A_A block copolymers
d)B_B_B block copolymers
12.Which of the following is correct statement regarding sorbitan esters?
a)Products of the esterification of a sorbitan with a fatty acid
b)Low HLB number, insoluble in water and used as W/O emulsifiers
c)Both of these
d)None of these
13.Which of the following is correct statement regarding polysorbates?
a)Ethoxylated derivatives of sorbitan esters
b)High HLB number, soluble in water and used as O/W emulsifiers
c)Both of these
d)None of these
14.Sorbitan esters are
a)Spans
b)Tweens
c)Both of these
d)None of these
15.Polysorbates are
a)Spans
b)Tweens
c)Both of these
d)None of these
Solutions:
- c)Both of these
- b)Adsorption
- d)CMC
- d)All of the above
- d)All of the above
- a)Benzethonium chloride
- b)Parenterals
- c)Both of these
- b)Sorbitan esters
- d)Polymeric surfactants
- a)A–B–A block copolymers
- c)Both of these
- c)Both of these
- a)Spans
- b)Tweens
References:
1. GAURAV KUMAR JAIN – THEORY & PRACTICE OF PHYSICAL PHARMACY, 1st edition 2012 Elsevier, page no. 99-103.
2. Martins Physical pharmacy, 6th edition 2011, page no. 670-675.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test