Career as Clinical Research Coordinator for Pharmacy and life sciences Graduates- High Growth Prospective
What is a clinical research coordinator?
Clinical research and drug trials, including interventions employing medications, technology, or processes encouraging behavioural changes, are overseen by clinical research coordinators, sometimes occasionally referred to as clinical trial managers. In this role, your responsibilities would include participant recruitment and screening, trial management, data collection, and report writing.
In addition to overseeing trial safety, a clinical research coordinator is in charge of maintaining the safety of study materials both during and after the study. You oversee adherence to rules and moral principles and monitor the well-being and advancement of patients.
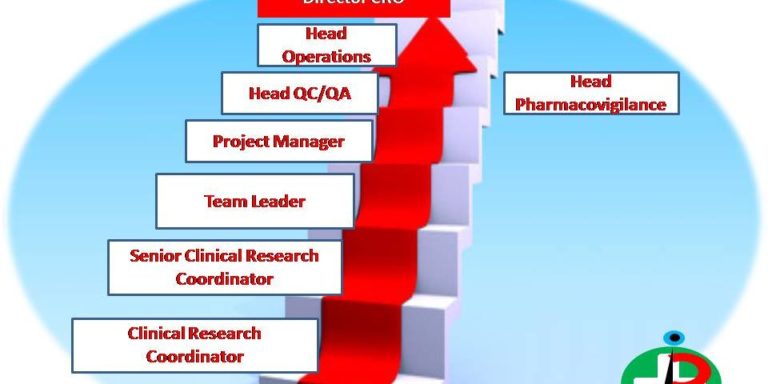
In a clinical research team, where does the clinical research coordinator fit in?
A primary investigator oversees a clinical research coordinator and is in charge of the study’s overall management and design. A clinical researcher, on the other hand, plans the daily operations of the trials. In addition, you will collaborate directly with sponsors and employees in their compliance, personnel, and finance department as a clinical research coordinator. Typically, a clinical research team consisting of physicians, nurses, other medical personnel, and assistance will be under your direction.
Want to peruse career in Clinical Data Management- fill the below form
What are the main tasks and obligations of a coordinator for clinical research?
Clinical research coordinators work in a variety of settings, including hospitals and private companies. The tasks you perform will depend on your workplace and the projects you are working on. Nonetheless, common duties include of:
- Managing and supervising clinical trial operations.
- Enlisting and vetting study participants.
- Keeping track of participant and trial documentation.
- Ensuring that trials are conducted in accordance with legal, ethical, and governmental guidelines.
- Guaranteeing the materials, supplies, and participants’ safety.
- Doing cost analyses and creating financial plans.
- Creating and delivering instruction to participants and employees.
- Gathering information and keeping thorough records.
- Serving as a point of contact for any queries or worries that study participants may have.
Education: a bachelor’s degree
To become a clinical research coordinator, one must typically have a bachelor’s degree. It is advised to have a Bachelor of Pharmacy, Science in a related field, such as clinical research administration, public health, microbiology, or any topic falling under the purview of the health sciences.
Salary
Clinical Research Coordinator salary in India ranges between ₹ 2.8 Lakhs to ₹ 7.2 Lakhs with an average annual salary of ₹ 3.6 Lakhs. This salary is based on survey by various organizations and salary may differ from the above mention data.
Certification Courses
If you wish to progress in your clinical research profession, obtaining a clinical research certification is advised but not necessary. You must be employed in the field in order to obtain certification. A certification attests to your abilities and proficiency, which may improve your pay and job opportunities. Clinical research workers can obtain a range of certifications from the Association of Clinical Research workers (ACRP-CP):
- ACRP-CP, or ACRP Certified Professional: To certify your proficiency in clinical research as a professional
- Coordinator for Clinical Research (CCRC): To certify your proficiency in clinical research coordination
- Clinical Research Associate (CCRA): To demonstrate your proficiency in the field, please complete this assessment.
- Principal Investigator (CPI): To certify you possess the necessary expertise and abilities,
CITI Programme also run various courses : Click to know more courses
ICRI India also run PG diploma in clinical research and Pharmacovigilance (PGDCRPV)
Get free courses information in clinical research & Pharmacovigilance – click here
Full guide of clinical research career
Careers in clinical research can take many different forms.
The following list of 10 clinical research occupations includes the duties associated with each position.
1. Clinical research coordinator (CRC) –A coordinator for clinical research (CRC) CRCs are entry-level employees that help with patient recruiting, getting informed permission, gathering data, and making sure protocols are followed. They keep track of paperwork, arrange research visits, and interact with the investigators.
2. Clinical research associate (CRA)- An associate in clinical research (CRA) Monitoring clinical trial sites, confirming data, making sure regulations and study protocols are followed, and evaluating the safety and well-being of study participants are all part of the duties of entry-level clinical research associate positions.
3. Clinical trial manager –Manager of clinical trials From the start of the study until its conclusion, clinical trial managers supervise every aspect of its operations. They oversee CRA teams, finances, and schedules to make that trials are carried out properly.
4. Clinical project manager-Project manager with clinical expertise Within a programme, clinical project managers coordinate and oversee several trials. They oversee resources, work with cross-functional teams, and make sure that every stage of a project is in line with corporate objectives.
5. Regulatory affairs specialist – A specialist in regulatory matters The administrative aspect of compliance is mostly the responsibility of regulatory affairs specialists. They gather, prepare, submit, and keep up-to-date regulatory paperwork for approval. You’ll need to communicate with regulatory bodies, remain up to date on new legislation, and make sure that all policies are followed.
6. Data manager – Information coordinator Clinical trial data is managed by data managers, who also supervise database management, data cleaning, and data gathering. They collaborate closely with biostatisticians to guarantee data quality and compliance with all regulations.
7. Clinical research scientist – A scientist in clinical research Scientists that conduct clinical research create study plans, gather and examine data, and interpret findings. In addition, they produce research reports and publish their findings in academic publications.
8. Medical monitor – Health-related monitor Medical monitors keep an eye on participant and patient safety throughout the trial, evaluate adverse occurrences, and offer modifications or study terminations based on their expertise in medicine.
9. Clinical quality assurance auditor –An auditor for clinical quality assurance In order to comply with rules and quality requirements, auditors do routine audits and inspections. They pinpoint instances of non-compliance and provide remedies.
10. Clinical research consultant – Consultant for clinical research Consultants offer professional advice on a range of clinical research topics, including as data analysis, trial design, and regulatory tactics. They collaborate with groups or work alone to find solutions to challenging issues.