Career in Clinical Data Management for Pharmacy and Lifesciences Graduates with good growth in future
What is Clinical Data Management?
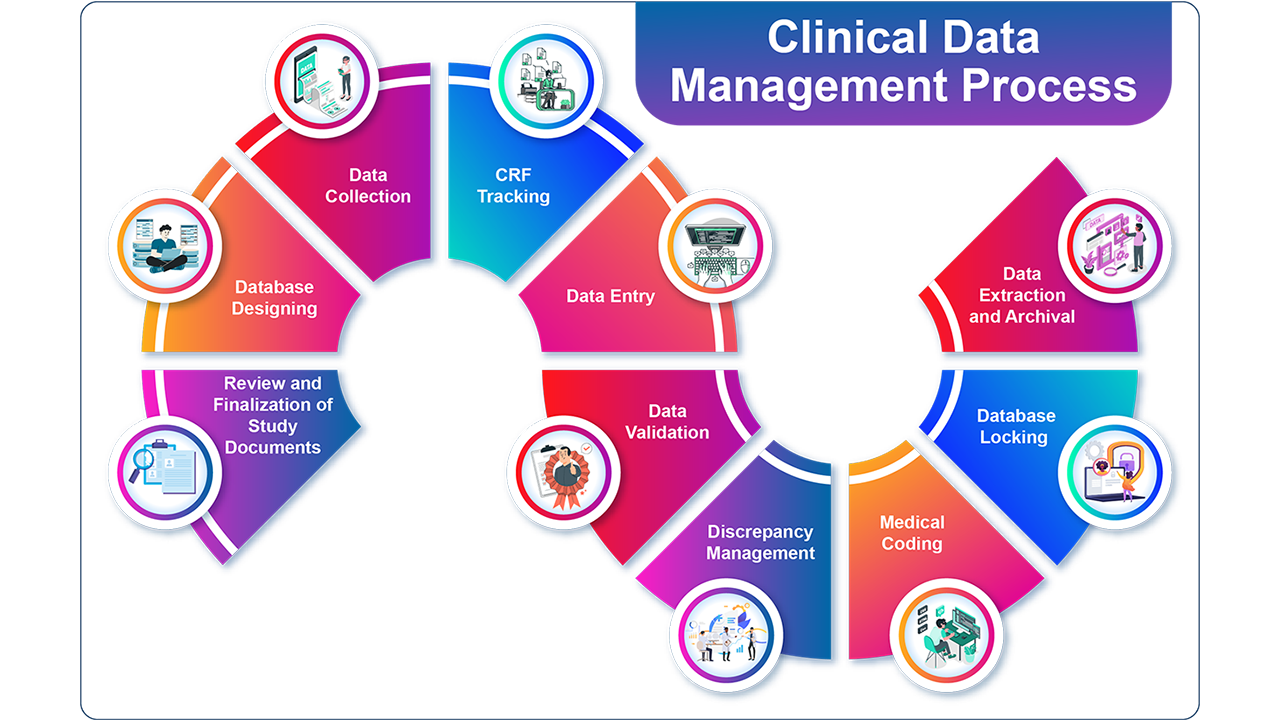
Clinical data management (CDM), which entails gathering, organising, and managing clinical trial data in compliance with legal requirements and industry best practices, is an essential step in the clinical research process. Clinical trial data must meet the highest requirements of data integrity and confidentiality, and CDM guarantees that the data is accurate, complete, and dependable.
A variety of tasks are involved in CDM, such as building study databases, designing case report forms (CRFs), developing data validation protocols, managing data queries, bringing disparate data sets together, and preparing clinical data for statistical analysis. Overseeing these duties and collaborating closely with other clinical research team members is the responsibility of a clinical data manager.
Clinical data managers use a variety of tools and technology, like as data visualisation and analysis software, clinical data management systems (CDMS), and electronic data capture (EDC) systems, to carry out these activities. Additionally, they need to be conversant with pertinent industry standards and regulatory norms, such as the International Council for Harmonisation (ICH) recommendations and Good Clinical Data Management Practices (GCDMP).
Ensuring that clinical trial data is of the greatest quality and integrity and that it can be utilised to support decision-making throughout the drug development process is the overall aim of clinical data management.
What Skills Needed for CDM?
- Strong precision and attention to detail.
- The capacity to function both alone and together.
- Excellent communication abilities.
- Good familiarity with clinical trial procedures and medical jargon.
- Competence with EDC systems.
- Comprehension of security and data privacy laws.
Education Required
- Bachelor’s degree in Pharmacy OR life sciences or related field
- Master’s degree or certification in clinical research, data management, or Health informatics related field can be a plus
Entry-level positions and their responsibilities:
Entry-level positions in clinical data management include roles such as Clinical Data Associate (CDA), Clinical Data Coordinator (CDC), and Clinical Data Entry Specialist. These positions typically require a Bachelor’s degree in a related field such as life sciences, computer science, or healthcare, although some employers may also accept candidates with an Associate’s degree and relevant experience.
Clinical data management entry-level jobs may involve the following duties:
- Gathering information from clinical trial locations and entering it into electronic data capture (EDC) systems.
- Checking the accuracy and completeness of the data.
- Resolving differences in data with research staff.
- Helping with quality control and data cleaning tasks.
- Creating and evaluating reports and data listings.
- Assisting in the creation of research documentation, including specifications for data validation and data management strategies.
- Assisting with the upkeep of tracking systems and study databases.
- These positions offer a strong foundation in clinical data management and could open doors for professional development and success in the industry.
Want to peruse career in Clinical Data Management- fill the below form
Options for career growth in CDM: The discipline of clinical data management offers a number of options for career progression. A handful of the positions are listed below.
1.Manager or Coordinator for Clinical Data: A clinical data coordinator works closely with clinical research workers and study investigators to ensure that all data is collected accurately and on time. They are also in charge of organising the management and reporting of clinical data.
2. Senior Clinical Data Manager: In this role, you will oversee the work of a group of clinical data managers and manage them. You will also be in charge of making sure that all clinical data is appropriately collected, maintained, and reported.
3.Clinical Data Scientist: The job of a clinical data scientist is to use statistical and analytical techniques to analyse clinical data in order to find trends and patterns that can guide the development of new drugs and patient care.
4.Project Manager: A clinical data management project manager is responsible for supervising the development, execution, and monitoring of clinical data management initiatives, guaranteeing their timely, cost-effective, and high-quality completion.
5.Clinical Data Quality Manager: The job of a clinical data quality manager is to ensure the accuracy and completeness of clinical data by creating and implementing quality control methods and keeping an eye on the data.
6.Director of Clinical Data Management: In an organisation, the director of clinical data management is in charge of managing personnel, creating policies and procedures, and making sure that all clinical data is accurately gathered, handled, and reported.
Other comparable positions into which one could move:
Clinical Data Scientist: To analyse and get insights from the vast amounts of data collected during clinical trials, clinical data scientists work with them. To find patterns and trends in the data, they might employ machine learning algorithms and statistical methods.
Clinical Trial Manager: Organising, coordinating, and carrying out clinical trials are the responsibilities of clinical trial managers. They collaborate closely with sponsors, investigators, and other relevant parties to guarantee that the study is carried out in compliance with deadlines and regulatory standards.
Biostatistician: Biostatisticians examine data from clinical trials and other medical research projects using statistical techniques. To make judgements regarding the effectiveness and safety of treatments, they create study protocols, carry out statistical analysis, and evaluate the findings.
Regulatory Affairs Specialist: The job of regulatory affairs specialists is to make sure that clinical trials meet all applicable regulations, including those established by the FDA. They ensure that the trial is carried out in compliance with relevant legislation, create and submit regulatory documentation, and communicate with regulatory agencies.
Clinical Research Associate: In order to verify that the study is carried out in compliance with the study protocol and regulatory requirements, clinical research associates, or CRAs, collaborate with clinical trial sites to track the trial’s progress. As well as these duties, they can choose the study site, start the study, and check the quality of the data.
CDM training Courses
The skills required for clinical data management can be developed through a variety of training programmes and courses. On the other hand, avoid enrolling in programmes that are not approved by corporate. These are a handful of genuine programmes in which you can enroll.
1. The Association of Clinical Research Professionals (ACRP) provides a range of in-person and virtual training programmes on data management and clinical research.
Courses: Introduction to Clinical Trials (acrpnet.org). Understanding clinical trial protocols: important factors to take into account for efficient development and feasibility review
2. The Society for Clinical Data Management (SCDM) provides numerous clinical data management-related webinars, seminars, and credential programmes. Go here to download the GCDMP Guide. This book provides comprehensive instruction on clinical data management.
3.Clinical Data Interchange Standards Consortium (CDISC): CDISC offers webinars and training sessions on the application and standards of CDISC, as well as guidelines for the gathering, evaluating, and reporting of clinical data. The following links will teach you about CDISC.
4. Coursera: Coursera offers a range of online courses on data management, programming, and statistics that can be useful for clinical data management.
5. ICRI-INDIA: this is oldest private Institute for clinical research it run various course on clinical research, Pharmacovigilance etc: Click Here to know more
6.Among the other resources is edX, which provides online courses on programming, statistics, and data analysis that are relevant to the management of healthcare data.
Udemy: A range of online courses on statistics, programming, and data management are available on Udemy and may be helpful for clinical data management.
LinkedIn Learning: This platform provides online courses on a range of clinical data management subjects, such as project management, data analysis, and programming.
Salary of CDM Professionals in India:
Clinical Data Manager salary in India ranges between ₹ 2.3 Lakhs to ₹ 10.5 Lakhs with an average annual salary of ₹ 6.2 Lakhs.