Comparative observational studies –Cohort Study | Pharmacovigilance Notes and Lecture
Cohort, cross sectional, and case-control studies are often referred to as observational studies because the investigator simply observes. No interventions are carried out by the investigator. With the recent emphasis on evidence based medicine and the formation of the Cochrane Database of randomised controlled trials, such studies have been somewhat glibly maligned.
However, they remain important because many questions can be efficiently answered by these methods and sometimes they are the only methods available. The objective of most clinical studies is to determine one of the following—prevalence, incidence, cause, prognosis, or effect of treatment; it is therefore useful to remember which type of
study is most commonly associated with each objective
While an appropriate choice of study design is vital, it is not sufficient. The hallmark of good research is the rigor with which it is conducted. Every published study should contain sufficient information to allow the reader to analyse the data with reference to these key points.
COHORT STUDIES
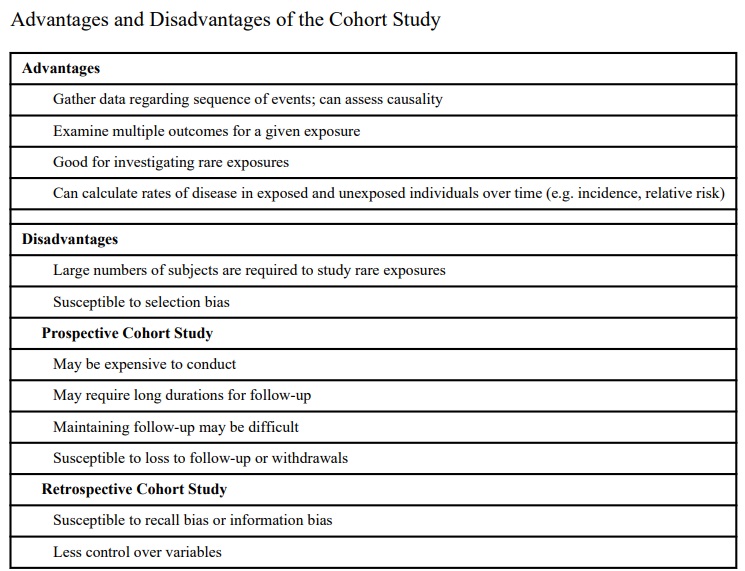
These are the best method for determining the incidence and natural history of a condition. The studies may be prospective or retrospective and sometimes two cohorts are compared.
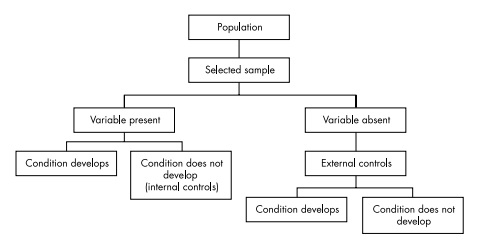
Prospective cohort studies A group of people is chosen who do not have the outcome of interest (for example, myocardial infarction). The investigator then measures a variety
of variables that might be relevant to the development of the condition. Over a period of time the people in the sample are observed to see whether they develop the outcome of interest (that is, myocardial infarction).
In single cohort studies those people who do not develop the outcome of interest are used as internal controls.
Where two cohorts are used, one group has been exposed to or treated with the agent of interest and the other has not, thereby acting as an external control.
Retrospective cohort studies These use data already collected for other purposes. The methodology is the same but the study is performed posthoc. The cohort is
“followed up” retrospectively. The study period may be many years but the time to complete the study is only as long as it takes to collate and analyse the data
How to run a cohort study
If the data are readily available then a retrospective design is the quickest method. If high quality, reliable data are not available a prospective study will be required.
The first step is the definition of the sample group. Each subject must have the potential to develop the outcome of interest (that is, circumcised men should not be included in a cohort designed to study paraphimosis). Furthermore, the sample population must be representative of the general population if the study is primarily looking at the incidence and natural history of the condition (descriptive).
If however the aim is to analyse the relation between predictor variables and outcomes (analytical) then the sample should contain as many patients likely to develop the outcome as possible, otherwise much time and expense will be spent collecting information of little value.
Each variable studied must be accurately measured. Variables that are relatively fixed, for example, height need only be recorded once. Where change is more probable, for
example, drug misuse or weight, repeated measurements will be required.
To minimise the potential for missing a confounding variable all probable relevant variables should be measured. If this is not done the study conclusions can be readily criticised. All patients entered into the study should also be followed up for the duration of the study. Losses can significantly affect the validity of the results. To minimise this as much information about the patient (name, address, telephone, GP, etc) needs to be recorded as soon as the patient is entered into the study.
Regular contact should be made; it is hardly surprising if the subjects have moved or lost interest and become lost to follow up if they are only contacted at 10 year intervals!
Beware, follow up is usually easier in people who have been exposed to the agent of interest and this may lead to bias.