VINBLASTINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
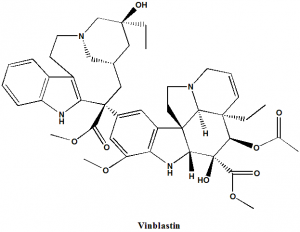
Vinblastine
IUPAC nomenclature
Dimethyl (2β,3β,4β,5α,12β,19α)-15-[(5S,9S)-5-ethyl-5-hydroxy-9-(methoxycarbonyl)-1,4,5,6,7,8,9,10-octahydro-2H-3,7-methanoazacycloundecino[5,4-b]indol- 9-yl]-3-hydroxy-16-methoxy-1-methyl-6,7-didehydroaspidospermidine-3,4-dicarboxylate.
Classification
Vinblastine falls under the category of Vinca alkaloids cytotoxic drugs.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 811 g/mol |
| 2 | Appearance | Solvated needles from methanol |
| 3 | Melting point | 267 °C |
| 4 | Solubility | Insoluble in water, soluble in organic solvents. |
| 5 | Octanol water partition coefficient | 3.7 |
| 6 | Presence of ring | Indole ring system
Piperidine ring Quinoline ring system |
Mechanism of Action
i. The primary activity of this drug is through inhibition of the mitosis at the metaphase stage of the cell by interaction with the tubuline protein.
ii. The drug leads to crystallization of the microtubule by binding with the microtubular proteins.
iii. This results in the mitotic arrest and cell death. [1]
Structural Activity Relationship
- Aryl or heteroaryl compounds decreases the activity of the drug.
- Strongly polar or charged groups also decreases the activity of the drug.
- Decreasing in the polarity of the substituent produces more active analogues of the drug.
- Decreasing the charge on the substituent also increases the activity of the analogues of the drug.
- The activity of the drug can be restores by the introduction of the lipophillic liker such as thioether.
- Small and uncharged molecules increase the activity and shows best results. [2]
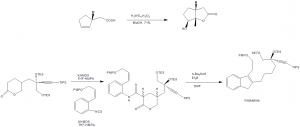
Methods of Synthesis
i. Alkaloids present in the Catharanthus roseus plant are extracted and separated into alkaloid tartrate with the help of benzene.
ii. Vinblastine is separated through the process of chromatography on aluminium oxide deactivated with acetic acid.
Chemical synthesis method:
i. Oxidative lactonization to produce the 5,5-fused γ-lactone.
ii. Lactone in deprotonated and isothiocynate is added to give thioanilide.
iii. This do radical mediated cyclisation onto the styrene to give indole.
iv. The indole is chlorinated with t-BuOCl to produce a reactive intermediate.
v. The intermediate so formed is coupled with the Vindoline to produce Vinblastine.
Therapeutic Uses
- Hodgkin’s and non-Hodgkin’s lymphoma
- Testicular cancers
- Breast cancers
- Lung cancers
- Head and neck cancers
- Melanomas
- Soft tissue sarcomas
- Germ cell tumors
- Fibromatosis
- Choriocarcinoma
- Kaposi’s sarcoma
- Mycosis fungoids
- Blood disorders
Side Effects
- Low blood counts, fatigue, weakness and reactions at the site of injection are common side effect
- Less common side effects include nausea, vomiting, loss of appetite, peripheral neuropathy, constipation, diarrhea, fever, loss of hair, loss of hearing, mouth sores, taste changes, shortness of breath, joint and muscle pain, tiredness, hypertension, depression, headache, etc.
MCQs
Q.1 Cytoblastin is the term related with which drug?
a) Vinblastin
b) Vincristin
c) Docetaxel
d) Paclitaxel
Q.2 Predict the incorrect statement related to the therapeutic uses of drug Vinblastin-
a) It is used for the treatment of some blood disorders like histiocytosis
b) It is used to cure the bone marrow depression effects
c) It is used for the treatment of Kaposi’s sarcoma
d) Fibromatosis can also be treated with help of this drug
Q.3 Match the following with respect to the SAR of drug Vinblastine
| i. Heteroaryl compounds | A. Increases the activity of drug |
| ii. Small and uncharged molecules | B. Activity of the drug can be restrored |
| iii. Introduction of the lipophillic linkers | C. Decreases the activity of drug |
| iv. Strongly polar groups | D. Decreases the activity of drug |
a) i-C, ii-B, iii-A, iv-D
b) i-A, ii-D, iii-B, iv-C
c) i-C, ii-A, iii-B, iv-D
d) i-B, ii-C, iii-A, iv-D
Q.4 Which amongst the following drugs shows its effect through interaction with tubuline proteins?
a) Vincristine
b) Vinblastine
c) Thiotepa
d) Both a) and b)
Q.5 Vinblastine drug belongs to which class?
a) Antibiotic
b) Vinca alkaloids
c) GnRH analogues
d) Folate antagonist antimetabolite
Q.6 Which of the following is not a side effect of Vinblastine?
a) Hair loss
b) Muscle and joint pain
c) Low blood counts
d) Hypotension
Q.7 Which amongst the following drugs is having least number of ring system in its structure-
a) Vinblastine
b) Morphine
c) Vincristine
d) Cyclophosphamide
ANSWERS
1-a
2-b
3-c
4-d
5-b
6-d
7-c
REFERENCES
[1] Himes RH, Kersey RN, Heller-Bettinger I, Samson FE. Action of the vinca alkaloids vincristine, vinblastine, and desacetyl vinblastine amide on microtubules in vitro. Cancer Research. 1976 Oct 1;36(10):3798-802. [2] Voss ME, Ralph JM, Xie D, Manning DD, Chen X, Frank AJ, Leyhane AJ, Liu L, Stevens JM, Budde C, Surman MD. Synthesis and SAR of vinca alkaloid analogues. Bioorganic & medicinal chemistry letters. 2009 Feb 15;19(4):1245-9.