6-MERCAPTOPURINE SAR, synthesis, mechanism of action, therapeutic actions, side effects and mcq
6-Mercaptopurine (6-MP)
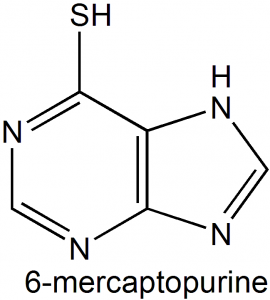
IUPAC nomenclature
3,7-dihydropurine-6-thione
Classification
6-Mercaptopurine falls under the category of purine antagonist antimetabolite.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 152.18g/mol |
| 2 | Appearance | Yellow to dark yellow crystalline powder |
| 3 | Melting point | 313 °C |
| 4 | Solubility | 6848 mg per 1000 ml of water |
| 5 | Octanol water partition coefficient | 0.01 |
| 6 | Presence of ring | Purine ring system |
Mechanism of Action
i. Mercaptopurine competes with hypoxanthine and guanine which are purine derived structures, for the HGPRT enzyme. Mercaptoporine is then converts into thio inosin monophosphate.
ii. Thi inosine monophosphate (TIMP) inhibits conversion of inosinic acid to xanthylic acid and adenylic acid through adenyl succinate.
iii. Methylation of thio inosine monophosphate forms 6-methylthioinosinate (MTIMP).
iv. Glutamine-5-phosphoribosylpyrophosphate amidotransferase is the enzyme requires for the purine ribonuceoltide synthesis. This enzyme is inhibited by thio inosine monophosphate and MTIMP.
v. Since, Glutamine-5-phosphoribosylpyrophosphate amidotransferase is rate limiting factor for purine synthesis, this alres the synthesis and functioning of the RNA and DNA.
vi. Thus, 6-Mercaptopurine interferes with synthesis of glycoprotiens and interconversion of nucleotides. [1]
Structural Activity Relationship
- The activity of the drug increases with increase in the carbon chain upto 15-16 carbons, after that, it again decreases.
- Substituent at position 6 which can lead to the increase in the resonance at 6th position will lead to increase in the activity of the drug.
- Introduction of the hydrophobic substituent at 6th position will increase the activity of the drug.
- Substitutions at 2nd position may not change the activity of the drug, or it may decrease the activity of the drug depending upon the type of substituent. [2]
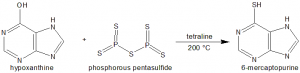
Methods of Synthesis
In the presence of tetraline as a solvent, hypoxanthine is heated along with excess of phosphorous pentasulfide. These are heated at 200 °C for a few hours.
Therapeutic Uses
- Acute lymphoblastic leukemia
- Ulcerative colitis
- Crohn’s disease
Side Effects
- Low blood counts and liver toxicity are common side effects of this drug.
- Other side effects can be nausea, vomiting, mouth sores, infertility, poor appetite, skin related problems like rashes, darkening of skin and diarrhea.
MCQs
Q.1 The terms ‘3,7-dihydropurine-6-thione’ and ‘purinethol’ are associated with which drug?
a) Methotrexate
b) 6-Thioguanine
c) 5-Flourouracil
d) 6-Marcaptopurine
Q.2 Activity of 6-Mercaptopurine can be increased through
a) Increase in the alkyl chain length
b) Decrease in the alkyl chain length
c) Hydrophilic substitution at 6th position
d) Any substitution at 2nd position
Q.3 Which amongst the following are the correct side effects of the drug ‘6-MP’?
I. Mouth sores
II. Liver toxicity
III. Ulcerative colitis
IV. Low blood counts
a) I, II, III & IV
b) I, II & IV
c) I, III & IV
d) II, III & IV
Q.4 The starting chemicals required for the synthesis of drug 6-Mercaptopurine are?
a) Hypoxanthine and phosphorous pentasulfide
b) Guanine and phosphorous pentasulfide
c) Hypoxanthine and copper sulfate pentahydrate
d) Guanine and copper sulfate pentahydrate
Q.5 Which pairs of drug and its classification are correct
| I. | BCNU | Antiandrogen |
| II. | DTIC | Triazine |
| III. | Mtx | Folate antagonist |
| IV. | 6-MP | Pyrimidine antagonist |
a) I & IV
b) II & III
c) I & III
d) IV & II
Q.6 Correct physical form in which the drug 6-Mercaptopurine is found at NTP?
a) Yellow buff powder
b) Brown to red colored powder
c) Yellow crystalline powder
d) Green colored liquid
Q.7 Match the following with respect to ring systems of the drug
| i. Mechlorethamine | A. Pteridine ring system |
| ii. 6-MP | B. Oxazophospharine ring |
| iii. Cyclophosphamide | C. No ring system present |
| iv. Methotrexate | D. Purine ring system |
a) i-A, ii-B, iii-C, iv-D
b) i-D, ii-B, iii-A, iv-C
c) i-B, ii-A, iii-D, iv-C
d) i-C, ii-D, iii-B, iv-A
ANSWERS
1-d
2-a
3-b
4-a
5-b
6-c
7-d