Clinical Trials Phases | Differences in various phases of Clinical Trials | Pharmacovigilance Notes & Lecture
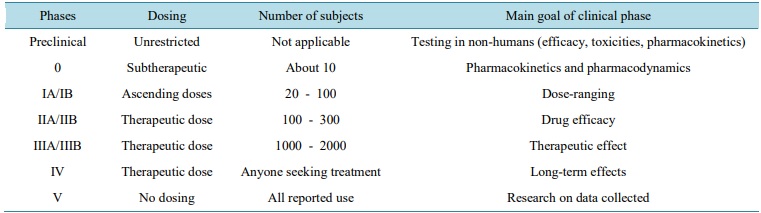
Clinical trial phases are steps in the research to determine if an intervention would be beneficial or detrimental to humans and include Phases 0, I, II, III, IV, clinical studies . During Phase 0, pharmacodynamics and pharmacokinetics are determined. Safety studies are evaluated during Phase I, efficacy during Phase II, and confirmation of safety and efficacy during Phase III. Sentry studies are done in Phase IV.
The different clinical trial phases are described in further detail below .
Phase 0
The official name of a Phase 0 study is an exploratory IND study, and the goal is to quickly establish whether an agent will work as desired in humans, based on in vivo safety pharmacology and toxicology preclinical studies. In Phase 0 studies, a single subtherapeutic dose of the IND is administered to a small number of healthy subjects (c.10 to 15), over a short duration (7 days). Since the dose administered is too low to result in a therapeutic effect (ensuring the absence of toxic effects), preliminary pharmacokinetic (PK) and, where possible, pharmacodynamic (PD) data are collected for evaluation.
Phase 1
Phase 1 studies are designed to assess the safety of an IND, to understand its PK and PD properties, and to ideally identify a potential therapeutic dose. These studies are usually conducted in a small number of healthy volunteers/subjects (c.15 to 30).
Phase 2
These studies are typically conducted to test the IND in a larger group of patients who have the disease or illness for which the IND is being developed, to determine whether it is efficacious, at least in the short term. Phase 2 studies are larger than those conducted earlier in the drug development, typically comprising up to 300 patients.
Phase 3
Phase 3 studies are designed and performed to assess the efficacy and effectiveness of an IND in a larger cohort of patients, all of whom have the disease that the treatment is intended to treat. Such studies are typically conducted in several hundred patients, and are usually conducted at multiple sites in multiple countries. Phase 3 studies often compare the new treatment versus the current ‘gold standard’ treatment for the condition for which the new treatment is being developed.
Phase 4
Post marketing surveillance involves monitoring for safety (pharmacovigilance) once a treatment has been approved by the appropriate regulatory authority or authorities. Such surveillance is intended to identify any rare adverse effects that have not been observed previously or have only been observed infrequently, and to monitor the effects of long term administration in a wider population.
Comparison of Clinical Trial Phases : Click Here to get PDF notes
See below table