ANASTROZOLE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
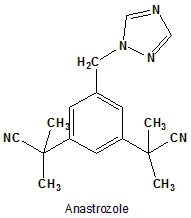
Anastrozole
IUPAC nomenclature
2,2′-[5-(1H-1,2,4-triazol-1-ylmethyl)-1,3-phenylene]bis(2-methylpropanenitrile).
Classification
Anastrozole is an aromatase inhibitor. [1]
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 293.4 g/mol |
| 2 | Appearance | Off white powder form |
| 3 | Melting point | 82°C |
| 4 | Solubility | 0.53mg/ml in water |
| 5 | Octanol/water partition coefficient | 1.58 |
| 6 | Presence of ring | Aryl and triazole ring present |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
i. Aromatase enzymes are competitively inhibited by the drug.
ii. This leads to prevention of conversion of androgens to estrogen.
iii. Due to reduced availability of estrogen, estrogen –dependent tumors regress.
Structural Activity Relationship
- At least a carbonyl group at C-3 or C-17 is required. It will be better if both are present. If one of the carbonyl group is required to be loosed then, it will be preferred to keep the C-17 carbonyl group.
- Enough planarity should be given in A-ring and A,B-ring junction by providing double bonds or other functions. Planarity is important for the inhibition of aromatase.
- Extend and conjugate the number of double bonds in convenient positions of A and B rings of the steroid at positions C-1, C-4 and C-6 can increase the potency of the drug.
- Bulky groups decrease the activity or binding of the drug.
- Substitutions at C-6 and C-7 positions can increase the aromatase inhibition property. [2]
Method of synthesis
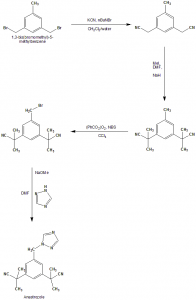
i. 3,5-bis(bromomethyl)toluene undergoes SN2 displacement reaction using potassium nitrile and tetrabutylammonium bromide as a phase transfer catalyst to give bis-nitrile compound.
ii. Bis-nitrile compound formed undergoes deprotonation with NaH and methylated afterwards with methyl iodide to give bis-dimethyated product.
iii. Product undergoes radical substitution reaction following the Wohl-Ziegler reaction using N-bromosuccinamide and benzoyl peroxide as the radical initiator.
iv. In the final step, benzylbromide undergoes SN2 displacement with sodium triazole to give anastrozole.
Therapeutic Uses
The drug used for the treatment of:
- Breast cancer in postmenopausal women.
Side Effects
- Common side effects of Anastrozole include stomach upset, hot flashes, muscle and joint pain.
- Less common side effects are increases cough, difficulty in breathing, peripheral edema, headache, trouble in sleeping, back pain, fractures, weakness in bones, rash, depression, high blood pressure, sore throat, mood disturbances, decreased energy, nausea and vomiting.
MCQs
Q.1 What can be the correct IUPAC nomenclature of Anastrozole?
a) 2,2′-[5-(1H-1,2,4-triazol-1-ylmethyl)-1,3-phenylene]bis(2-methylpropanenitrile)
b) 2,2′-phenylene]bis(2-methylpropanenitrile)
c) 5-(1H-1,2,4-triazol-1-ylmethyl)-1,3-phenylene]bis(2-methylpropanenitrile)
d) 2,2′-[5-(1H-1,2,4-triazol-1-ylmethyl)-1,3-phenylene]
Q.2 Which amongst the following statements is/are incorrect related to the SAR of Anastrozole?
I. Carbonyl groups are not necessary for the activity of drug.
II. Substitutions at C-6 and C-7 positions can increase the aromatase inhibition property.
III. Extend and conjugate the number of double bonds in convenient positions of A and B rings of the steroid at positions C-1, C-4 and C-6 can decrease the potency of the drug.
a) I
b) I & II
c) I & III
d) I, II & III
Q.3 The correct order for the mechanism of action of Anastrozole can be?
I. Regress in estrogen receptor positive tumors.
II. Reduction in the conversion of androgens to estrogen.
III. Competitive inhibition of aromatase enzyme.
a) III – I – II
b) I – III – II
c) III – II – I
d) I – II – III
Q.4 The drug Anastrozole is mainly used for the treatment of?
a) Chronic obstructive pulmonary disease
b) Neuromascular disorder
c) Siezures
d) Breast cancer
Q.5 Match the drugs with the correct classification.
| i. Triptorelin | A. GnRH analogues |
| ii. Bicalutamide | B. Antiandrogens |
| iii. Anastrozole | C. Aromatase inhibitors |
| iv. Dactinomycin | D. Antibiotics |
a) i-A, ii-D, iii-C, iv-B
b) i-D, ii-B, iii-C, iv-A
c) i-A, ii-B, iii-C, iv-D
d) i-D, ii-C, iii-B, iv-A
Q.6 How many statements below are true with respect to the side effects of the drug Anastrozole?
- Hot flashes is a common side effect
- Stomach upset is a common side effect
- Insomia is a less common side effect
- Osteoporosis is a less common side effect
a) 1
b) 2
c) 3
d) 4
Q.7 The type of ring system found in Anastrozole is?
a) Triazole ring
b) Pteridine ring
c) Oxazophospharine ring
d) None of the above
ANSWERS
1-a
2-c
3-c
4-d
5-c
6-d
7-a
REFERENCES
[1] Tripathi KD. Essentials of Medical Pharmacology, 6thEdn. Jaypee Brothers Medical Publishers (P) Ltd. 2008: 820. [2] Varela C. Design, synthesis and structure-activity relationships studies on steroidal aromatase and 5α-reductase inhibitors as anti-tumors (Doctoral dissertation).