AZATHIOPRINE Synthesis, SAR, MCQ,Structure and Therapeutic Uses
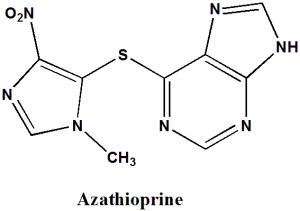
Azathioprine
IUPAC nomenclature
6-[(1-Methyl-4-nitro-1H-imidazol-5-yl)sulfanyl]-7H-purine
Classification
Azathioprine falls under the category of purine antagonist antimetabolite. It is also an immunosuppressive agent.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 277.27 g/mol |
| 2 | Appearance | Pale yellow crystalline powder |
| 3 | Melting point | 243.5 °C |
| 4 | Solubility | Insoluble in water. Soluble in dilute alkali solutions. |
| 5 | Octanol water partition coefficient | 0.1 |
| 6 | Presence of ring | Purine ring system |
Mechanism of Action
i. Azathioprine inhibits the synthesis of purine in the cell.
ii. This will further leads to the inhibition of synthesis of DNA and RNA.
iii. Azathioprine also interacts with cellular metabolism and results in the inhibition of mitosis. [1]
Structural Activity Relationship
- The activity of the drug increases with increase in the carbon chain upto 15-16 carbons, after that, it again decreases.
- Substituent at position 6 which can lead to the increase in the resonance at 6th position will lead to increase in the activity of the drug.
- Introduction of the hydrophobic substituent at 6th position will increase the activity of the drug.
- Substitutions at 2nd position may not change the activity of the drug, or it may decrease the activity of the drug depending upon the type of substituent. [2]
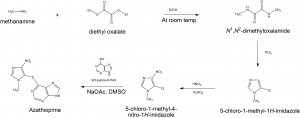
Methods of Synthesis
i. Methanamine is reacted with diethyl oxalate in the presence of ethylhydroxide at room temperature to form N,N’- dimethyloxalamide.
ii. N,N’-dimethyloxalamide is then treated with PCl5 to give 5-chloro-1-methyl-1H-imidazol.
iii. The formed compound undergoes nitration reaction to produce5-chloro-1-methyl-4-nitro-1H-imidazol.
iv. It is then treated with 9H-purine-8-thiol to finally produce Azathioprine.
Therapeutic Uses
- Used as an immunosuppressive agent for prevention of rejection of the transplanted organs.
It is given for the Treatment of
- Autoimmune diseases
- Rheumatoid arthritis
- Pemphigus
- Autoimmune hepatitis
- Myasthenia gravis
- As a steroid sparing agent for inflammatory bowel diseases.
Side Effects
- Nausea and vomiting are the common side effects.
- Hypersensituivity reactions, dizziness, diarrhea, fatigue, skin rashes and loss of hair can also be seen in the patient taking this drug.
- Low blood count may take place due to the suppression of the bone marrow of the patient.
- It can also cause acute pancreatitis.
- From various studies it was found that Azathioprine may induce cancers, most probably skin cancers.
MCQs
Q.1 What can be the correct IUPAC nomenclature of the drug Azathioprine
a) 6-[(1-Methyl-4-nitro-1H-imidazol-5-yl)sulfanyl]-7H-purine
b) 1,1′,1′′-Phosphorothioyltriaziridine
c) 4-[bis(2-chlorethyl)amino]benzenebutanoic acid
d) 3,7-dihydropurine-6-thione
Q.2 Which amongst the following statements is/are INCORRECT related to the SAR of Azathioprine?
I. Activity of the drug increases with decrease in the carbon chain length.
II. Substitution at the 2nd position can increase the activity of the drug irrespective to the type of substituent.
III. Introduction of the lipophillic group at the 6th position will increase the activity of the drug.
a) II & III
b) I & II
c) I, II & III
d) Only II
Q.3 The correct order for the mechanism of action of Azathioprine can be?
I. DNA and RNA synthesis are inhibited
II. Inhibition of purine synthesis
III. Inhibition of pyrimidine synthesis
IV. Cytotoxicity of cell
a) I-III-IV
b) I-II-IV
c) III-I-IV
d) II-I-IV
Q.4 The drug Azathioprine is mainly used for?
a) Treatment of skin cancers
b) As an immunosuppressant
c) Treatment of diabetes
d) Treatment of mouth sores
Q.5 Match the drugs with the correct classification.
| i. 5-Flourouracil | A. Purine antagonist antimetabolite |
| ii. Azathioprine | B. Pyrimidine antagonst antimetabolite |
| iii. Methotrexate | C. Folate antagonist antimetabolie |
| iv. Anastrozol | D. Aromatase inhibitor |
a) i-C, ii-D, iii-A, iv-B
b) i-D, ii-C, iii-A, iv-B
c) i-A, ii-B, iii-D, iv-C
d) i-B, ii-A, iii-C, iv-D
Q.6 How many statements below are true with respect to the side effects of the drug Azathioprine?
- Nausea and vomiting are common side effects.
- Low blood counts due to autoimmunation
- Cancer may occur to the patient taking this drug
- Acute pancreatitis may occur
a) 3
b) 1
c) 4
d) 2
Q.7 The type of ring system found in Azathioprine is?
a) Pyrimidine ring system
b) Purine ring system
c) Pteridine ring system
d) Both b) and c)
For More Standard and Quality Question Bank you can Join Our Test Series Programme for GPAT, NIPER JEE, Pharmacist Recruitment Exam, Drug Inspector Recruitment Exams, PhD Entrance Exam for Pharmacy: Click Here
ANSWERS
1-a
2-c
3-d
4-b
5-d
6-a
7-c