Carmustine (BCNU) Synthesis, SAR, MCQ,Chemical Structure and Therapeutic Uses
Carmustine (BCNU)
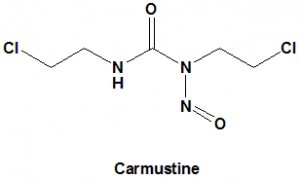
IUPAC nomenclature
1,3-Bis(2-chloroethyl)-1-nitrosourea
Classification
Carmustine falls under the category of alkyl Nitrosoureas alkalyting agents.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 214.05 g/mol |
| 2 | Appearance | Orange yellow solid |
| 3 | Melting point | 31°C |
| 4 | Solubility | 0.02 M in water; very soluble in ethanol |
| 5 | Octanol water partition coefficient | 1.53 |
| 6 | Presence of ring | No ring system |
Mechanism of Action
- Carmustine cross links the DNA strands through alkylation. This results in the disruption of DNA function and it leads to apoptosis of cell.
- Along with alkylation, Carmustine also carbomylates the proteins, which also includes the enzymes like DNA repair enzymes.
- It is highly lipophillic and can cross the blood brain barrier very easily. [1]
Structural Activity Relationship
- Binding with the amino group will increase the oral route availability of the drug
- The introduction of the substituted phenyl group will also increases the oral route availability of the drug.
- Aromatic ring introduction will increase the stability of the drug.
- Aromatic ring will further increase the distribution of the drug throughout the body.
- Benzimidazole ring can provide the local and faster action of the drug.
- Benzimidazol will further decrease the half life of compound.
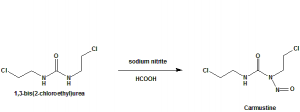
Methods of Synthesis
Carmustine is synthesized through nitrosation with sodium nitrite of 1,3-bis(2-chloroethyl)urea in an acidic and cold medium.
Therapeutic Uses
- Carmustine is used for the treatment of brain tumors, brainstem glioma, glioblastoma, metastatic brain tumors, medulloblastoma, ependymoma, astrocytoma and ependymoma
- Multiple mylomas, hodgkin’s disease and non-Hodgkin’s lymphomas can also be cured with Carmustine. [2]
Side Effects
- Nausea, vomiting and CNS effects are common.
- Facial flushing and low blood counts are also side effects of Carmustine.
- High doses of this drug may lead to pulmonary toxicity.
MCQs
Q.1 “1,3-Bis(2-chloroethyl)-1-nitrosourea” is the IUPAC nomenclature of which drug?
a) Cyclophosphamide
b) Carmustine
c) Dacarbazine
d) Methotrexate
Q.2 Predict the incorrect statement related to therapeutic uses of drug Carmustine
a) Carmustine is used for the treatment of low blood counts
b) Carmustine is used for the treatment of multiple myelomas
c) Carmustine is used for the treatment of brain tumors
d) Carmustine is used for the treatment of Non-Hodgkin’s lymphoma
Q.3 Match the following with respect to the SAR of drug Carmustine
| i. Binding with amino group | A. increase stability of the drug |
| ii. Benzimidazol ring introduction | B. increase the oral route availability |
| iii. Aromatic ring introduction | C. local and faster action |
| iv. Aromatic ring introduction | D. increase the distribution of drug throughout body |
a) i-A, ii-D, iii-C, iv-B
b) i-D, ii-C, iii-B, iv-A
c) i-C, ii-B, iii-D, iv-A
d) i-B, ii-C, iii-A, iv-D
Q.4 Which amongst the following drugs shows its effect through alkylation?
a) Carmustine
b) Lomustine
c) Dacarbazine
d) All of the above
Q.5 Carmustine drug belongs to which class?
a) Antibiotics
b) Taxanes
c) Purine antagonist
d) Alkylating agents
Q.6 Which of the following is not a side effect of drug Carmustine
a) Facial flushing
b) Low blood counts
c) Brain tumors
d) Pulmonary toxicity
Q.7 Which amongst the following drugs is having least number of ring system in its structure-
a) Lomustine
b) Carmustine
c) Dacarbazine
d) Morphine
ANSWERS
1-b
2-a
3-d
4-d
5-d
6-c
7-b
References
[1] Weiss RB, Issell BF. The nitrosoureas: carmustine (BCNU) and lomustine (CCNU). Cancer treatment reviews. 1982 Dec 1;9(4):313-30. [2] Dixit S, Hingorani M, Achawal S, Scott I. The sequential use of carmustine wafers (Gliadel®) and post-operative radiotherapy with concomitant temozolomide followed by adjuvant temozolomide: a clinical review. British journal of neurosurgery. 2011 Aug 1;25(4):459-69.