CYTARABINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
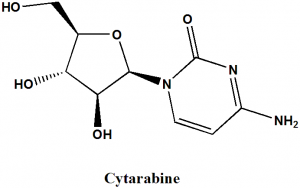
Cytarabine
IUPAC nomenclature
4-amino-1-[(2R,3S,4S,5R)-3,4-dihydroxy-5- (hydroxymethyl)oxolan-2-yl] pyrimidin-2-one
Classification
Cytarabine falls under the category of pyrimidine antagonist antimetabolite.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 243.22 g/mol |
| 2 | Appearance | Colorless crystalline powder |
| 3 | Melting point | 212.5 °C |
| 4 | Solubility | It is freely soluble in water |
| 5 | Octanol water partition coefficient | -2.8 |
| 6 | Presence of ring | Pyrimidine and a Tetrahydrofuran ring. |
Mechanism of Action
i. Cytarabine gets converted into active form cytarabine triphosphate by the action of deoxycytidine kinase within the cell.
ii. Competition of cytarabine triphosphate for the DNA polymerase enzyme inhibits the synthesis of DNA.
iii. Further, the drug produces cytotoxicity in the cell through incorporation into DNA and RNA.
iv. Cytarabine produces its effects mainly on the cell which are actively dividing by blocking the progression of the cell from G-1 phase to the S phase.
v. These overall results in the death of the actively dividing cells. [1]
Structural Activity Relationship
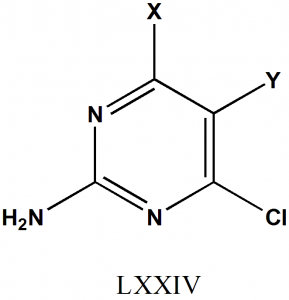
- The molar refractivity of the Y substituent will produce increase in the activity of drug.
- Substituent at Y with (CH2)3NRiR2 with R2 = COCH3, COC6H5, or COOCeH5 will produce a negative effect on the activity of the drug.
- SH at 4th position will increase the activity of drug.
- Substituent which are bulky in nature at Y position will produce negative effect on activity due to steric hindrances.
- Electron withdrawing groups at 5th position will increase the activity of drug. [2]
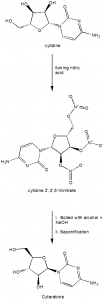
Methods of Synthesis
i. Cytidine and fuming nitric acid are reacted to form cytidine 2’,3’,5’-trinitrate.
ii. 2’,3’,5’-trinitrate is boiled in alcohol containing dilute NaOH which results in the formation of inverted 2’-hydroxy compound.
iii. The compound undergoes saponification for the removal of extra nitrate groups.
Therapeutic Uses
- Acute myelogenous leukemia
- Chronic mylogenous leukemia
- Acute lymphocytic leukemia
- Acute promyelocytic leukemia
- Hodgkin’s lymphoma
- Meningeal leukemia
- Cancers found in the lining of brain and spinal cord
Side Effects/Adverse event
- Common side effects includes diarrhea, nausea, vomiting, headache, mouth sores and low blood counts.
- Some other side effects which the patient may experience includes Diarrhea, Loss of appetite, Skin rashes, Hair thinning or hair loss, Eye pain, Flu-like symptoms, Dizziness, etc.
MCQs
Q.1 Which term is NOT associated with the drug Cytarabine?
a) 4-amino-1-[(2R,3S,4S,5R)-3,4-dihydroxy-5- (hydroxymethyl)oxolan-2-yl] pyrimidin-2-one
b) Cytosar-U
c) Alkeran
d) Cytabin
Q.2 Match the following with respect to the physical forms of drugs
| i. Azathiprine | A. Colorless crystalline powder |
| ii. Chlorambucil | B. White crystalline powder |
| iii. 5-FU | C. White granular powder |
| iv. Cytarabine | D. Pale yellow crystalline powder |
a) i-A, ii-B, iii-D, iv-C
b) i-B, ii-C, iii-A, iv-D
c) i-A, ii-B, iii-C, iv-D
d) i-D, ii-C, iii-B, iv-A
Q.3 The drug Cytarabine shows its action through
a) Producing osmotic imbalance in the cell
b) Competition for DNA polymerase enzyme
c) Leading the cell to the G0 phase
d) Membrane proliferation of the cell
Q.4 The correct order for the synthesis of the drug Cytarabine can be?
I. Boiling with alcohol in presence of alkali hydroxide
II. Reaction of Cytidine with Fuming Nitric acid
III. Boiling with alcohol in presence of Haloacids
IV. Saponification
a) II – I – IV
b) II – III – I – IV
c) II – III – IV
d) II – IV – III
Q.5 Predict the INCORRECT statements from the following with respect to the classification of the drug.
I. Chlorambucil can be classified as an alkylating agent.
II. Cytarabine falls under the category of purine antagonist antimetabolite.
III. Azathioprine falls under the category of purine antagonist antimetabolite.
IV. Fludarabine falls under the category of pyrimidine antagonist antimetabolite
a) I & III
b) II & IV
c) II & III
d) I & II
Q.6 The correct sequence of True and False for the given statements with respect to the side effects of drug Cytarabine is
I. Headache
II. Low blood counts
III. Eye pain
IV. Loss or thinning of hair
a) TTTF
b) TFTF
c) FTFT
d) TTTT
Q.7 Which amongst the following drugs is having highest number of ring system in its structure-
a) 5-Flourouracil
b) Clorambucil
c) Cytarabine
d) Mechlorethanamine
ANSWERS
1-c
2-d
3-b
4-a
5-b
6-d
7-c