ETOPOSIDE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Etoposide
IUPAC nomenclature
4′-Demethyl-epipodophyllotoxin 9-[4,6-O-(R)-ethylidene-beta-D-glucopyranoside], 4′ -(dihydrogen phosphate).
Classification
Etoposide falls under the category of epipodophyllo toxin cytotoxic drug.
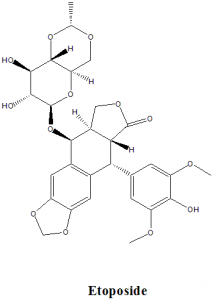
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 588.6 g/mol |
| 2 | Appearance | Present in solid form; crystal formation from methanol. |
| 3 | Melting point | Range between 236-251°C |
| 4 | Solubility | Sparingly soluble in water |
| 5 | Octanol water partition coefficient | 0.6 |
| 6 | Presence of ring | Ring structures like benzofurane, dioxole present |
Mechanism of Action
i. DNA topoisomerase is inhibited by Etoposide.
ii. Due to this, DNA re-ligation is inhibited.
iii. Inhibition of DNA re-ligation causes critical errors in DNA synthesis at the premitotic stage of cell division
iv. This leads to apoptosis of cell. [1]
Structural Activity Relationship
- Introducing the hydroxyl group at 2- and 4- positions of phenyl ring can increase the selectivity and potency of the drug.
- Compounds without the hydroxyl groups are found to be less active.
- Introduction of hydroxyl groups at ortho and para groups at 2-phenyl ring showed stronger topo II inhibitory activity
- Compounds having hydroxyl groups at meta position showed potent topo II inhibitory activity and strong antiproliferative activity.
- Overall, the hydroxyl groups at ortho and meta positions are important for the activity of the drug.[2]
Methods of Synthesis
i. Podophyllotoxin is treated with hydrogen bromide to give 1-bromo-1-deoxyepipodophyllotoxin.
ii. Compound formed is then demethylated to 1-bromo-4’-demethylepipodophyllotoxin.
iii. Bromine is replaced by hydroxy group to give 4’-demethylepipodophyllotoxin.
iv. Phenolic hydroxyl group is protected.
v. 4-hydroxy group is coupled with 2,3,4,6-tetra-O-acetyl-beta-D-glucopyranose.
vi. Protecting groups from the 4’-hydroxy group is removed by hydrogenolysis
vii. Acyl groups are removed by hydrolysis.
viii. The cyclic O-4,6 acetal is formed by reaction with acetaldehyde dimethyl acetal [3]
Therapeutic Uses
Etoposide is used for the treatments of:
- Testicular cancer
- Bladder cancer
- Prostate cancer
- Lung cancer
- Stomach cancer
- Uterine cancer
- Hodgkin’s lymphomas
- Non-Hodgkin’s lymphoma
- Mycoisis fungoids
- Kaposi’s sarcoma
- Wilm’s tumor
- Rhabdomyosarcoma
- Ewing’s sarcoma
- Neuroblastoma
- Brain tumors
Also given during bone marrow transplant setting.
Side Effects
- Common side effects includes low WBC count, low platelet count, loss of hair, menopause, loss of fertility, nausea, vomiting and low blood pressure..
- Some people may suffer from side effects mouth sores, diarrhea, loss of appetite, skin reactions inflammation at injection site, metallic tastes and peripheral neuropathy.
MCQs
Q.1 Match the related brand name of drugs:
| i. Melphalan | A. ALKERAN |
| ii. Mechlorethamine | B. ENDOXAN |
| iii. Thiotepa | C. MUSTINE |
| iv. Cyclophosphamide | D. MYLERAN |
a) i-B, ii-C, iii-D, iv-A
b) i-A, ii-D, iii-C, iv-B
c) i-D, ii-B, iii-C, iv-A
d) i-A, ii-C, iii-D, iv-B
Q.2 How many statements below are true with respect to the SAR of the drug etoposide?
- Hydroxyl groups are important for the activity of the drug.
- Introduction of the hydroxyl groups can increase the activity of the drug
- Introduction of hydroxyl groups at meta position of phenyl ring decreases the potency of the drug
a) 1
b) 2
c) 3
d) 0
Q.3 The drug Etoposide is found in which form when treated with methanol?
a) Gives amorphous powdery appearance
b) Green oily liquid
c) Solid crystal form
d) None of the above
Q.4 The incorrect with respect to the method of synthesis of the drug Etoposide is?
a) Podophyllotoxin is treated with hydrogen bromide to give 1-bromo-1-deoxyepipodophyllotoxin.
b) Podophyllotoxin is treated with bromine water to give 1-bromo-1-deoxyepipodophyllotoxin.
c) Acyl groups are removed by hydrolysis.
d) All the above statements are true
Q.5 Which amongst the following is not a therapeutic use of drug Etoposide?
a) Prostate cancer
b) Hodgkin’s lymphoma
c) Non-Hodgkin’s lymphoma
d) None of these
Q.6 The correct classification of the drug Etoposide can be?
a) Folate antagonist
b) Purine antagonist antimetabolite
c) Epipodophyllo toxin
d) Pyrimidine antagonist antimetabolite
Q.7 How many number of rings are found in the chemical structure of the drug etoposide?
a) 9
b) 10
c) 7
d) 8
ANSWERS
1-d
2-b
3-c
4-b
5-d
6-c
7-c
REFERENCES
[1] Van Maanen JM, Retel J, De Vries J, Pinedo HM. Mechanism of action of antitumor drug etoposide: a review. JNCI: Journal of the National Cancer Institute. 1988 Dec 7;80(19):1526-33. [2] Design, synthesis, and structure-activity relationships of new benzofuro[3,2- b ]pyridin-7-ols as DNA topoisomerase II inhibitors. [3] IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: http://monographs.iarc.fr/ENG/Classification/index.php, p. V76 180 (2000).