LOMUSTINE Synthesis, SAR, MCQ,Chemical Structure and Therapeutic Uses
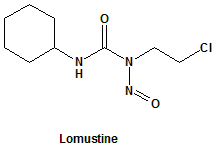
Lomustine (CCNU)
IUPAC nomenclature
1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea
Classification
Lomustine falls under the category of alkyl Nitrosoureas alkalyting agents.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 233.69 g/mol |
| 2 | Appearance | Solid yellow powder |
| 3 | Melting point | 89°C |
| 4 | Solubility | Freely soluble in chloroform, soluble in acetone |
| 5 | Octanol water partition coefficient | 2.83 |
| 6 | Presence of ring | Cyclohexyl ring |
Mechanism of Action
- Lomustine undergoes hydrolysis in vivo to convert into its metabolites.
- Metabolites causes alkylation and cross-linking at C6 position of guanine base pairs.
- It will induce cytotoxicity in the cell and it will lead to apoptosis of the cell.
- It also increases cytotxicity in the cell by inhibiting the protein modification through cabamylation action. [1]
Structural Activity Relationship
- Binding with the amino group will increase the oral route availability of the drug
- The introduction of the substituted phenyl group will also increases the oral route availability of the drug.
- Aromatic ring introduction will increase the stability of the drug.
- Aromatic ring will further increase the distribution of the drug throughout the body.
- Benzimidazole ring can provide the local and faster action of the drug.
- Benzimidazol will further decrease the half life of compound.
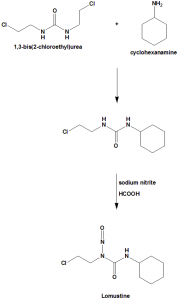
Methods of Synthesis
Carmustine is synthesized through nitrosation of 1-(2-chloroethyl)-3-cyclohexylurea in cold and acidic medium.
Therapeutic Uses
- Lomustine is used for the treatment of both, primary and metastatic brain tumors.
- It is also used for the treatment of Hodgkin’s disease and non-Hodgkin’s lymphomas.
- Colon cancer, lung cancer and melanomas are also treated with Lomustine.
Side Effects
- Nausea, vomiting and low blood counts are the common side effects.
- Hair loss, loss of fertility, mouth sores and loss of appetite are the less common side effects.
- High doses of Lomustine may cause Pulmonary toxicity and kidney toxicity. [2]
MCQs
Q.1 What can be the correct IUPAC nomenclature of Lomustine
a) 1-(2-chloroethyl)-3-cyclohexyl-2-nitrosourea
b) 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea
c) 1,3-Bis(2-chloroethyl)-1-nitrosourea
d) 1,3-Bis(2-chloroethyl)-2-nitrosourea
Q.2 Which amongst the following statements is/are incorrect related to the SAR of Lomustine?
I. The introduction of the substituted phenyl group will increases the oral route availability of the drug.
II. Aromatic ring introduction will decrease the stability of the drug.
III. Benzimidazole ring can provide the local and faster action of the drug.
a) I and III
b) I and II
c) II and III
d) Only II
Q.3 The correct order for the mechanism of action of Lomustine can be?
I. Apoptosis of cell
II. Alkylation of DNA
III. Hydrolysis of drug
IV. Conversion of drugs into its metabolites
a) III – IV – II – I
b) II – III – IV – I
c) I – III – IV – II
d) I – II – III – IV
Q.4 The drug Lomustine is mainly used for the treatment of?
a) Primary brain tumors
b) Metastatic brain tumors
c) Colon cancers
d) All of the above
Q.5 Match the drugs with the correct classification.
| i. Cytarabine | A. Folate antagonist |
| ii. Methotrexate | B. Nitrosoureas |
| iii. Lomustine | C. pyrimidine antagonist |
| iv. Mechlorethamine | D. Nitrogen mustard |
a) i-A, ii-C, iii-B, iv-D
b) i-D, ii-B, iii-A, iv-C
c) i-C, ii-A, iii-B, iv-D
d) i-B, ii-D, iii-C, iv-A
Q.6 How many statements below are true with respect to the side effects of the drug Lomustine?
- Nausea and vomiting are common side effects
- Even lower doses of drug can induce pulmonary toxicity
- Hair losses may occur after medication with Lomustine
- Kidney toxicity may occur on taking lower doses of drugs
a) 1
b) 2
c) 3
d) 4
Q.7 The type of ring system found in Lomustineis?
a) Cyclohexyl ring system
b) Aromatic ring system
c) Benzimidazol ring system
d) Both a) and c)
Answers
1-b
2-d
3-a
4-d
5-c
6-b
7-a
References
[1] Weiss RB, Issell BF. The nitrosoureas: carmustine (BCNU) and lomustine (CCNU). Cancer treatment reviews. 1982 Dec 1;9(4):313-30. [2] Pfreundschuh MG, Schoppe WD, Fuchs R, Pflüger KH, Loeffler M, Diehl V. Lomustine, etoposide, vindesine, and dexamethasone (CEVD) in Hodgkin’s lymphoma refractory to cyclophosphamide, vincristine, procarbazine, and prednisone (COPP) and doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD): a multicenter trial of the German Hodgkin Study Group. Cancer treatment reports. 1987 Dec;71(12):1203-7.