Penicillin G Synthesis, SAR, MCQ and Chemical Structure
Penicillin G
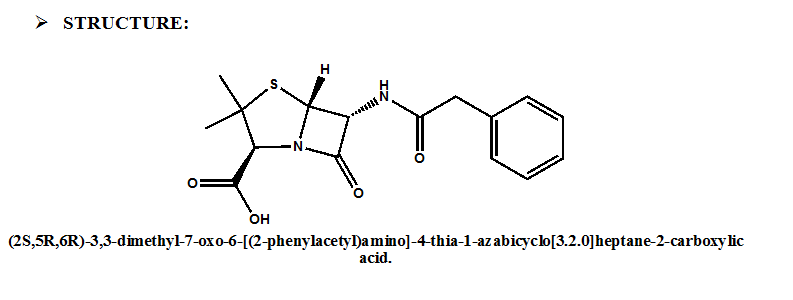
IUPAC NOMENCLATURE: (2S,5R,6R)-3,3-dimethyl-7-oxo-6-[(2-phenylacetyl)amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid. [1]
CLASSIFICATION: It is a Penicillin-class antimicrobial drug. When looking at its chemical classifications, penicillin g comes under Penicillins.
PHYSIOCHEMICAL PROPERTIES:
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 334.4 g/mol |
| 2 | Appearance | Solid amorphous white powder |
| 3 | Melting point | 214-217°C |
| 4 | Solubility | 210 mg/L in water at 25 °C |
| 5 | Dissociation constant (pKa) | 2.74 at 25°C |
| 6 | Presence of ring | β-lactam ring and thiazolidine ring, fused together. |
MECHANISM OF ACTION:
- β-lactam ring of the penicillin binds with enzyme DD-transpeptidase.
- DD-transpeptidase is essential for the crosslinkage during formation of peptidoglycan in bacterial cell wall.
- As a result, the formation of the cell wall in the bacteria disturbs and this further leads to the death of bacteria. [2]
STRUCTURAL ACTIVITY RELATIONSHIP
- C-6amino west end substitution:
- By introduction of amine group can increase the potency of drug
- Compounds contaiing the groups like sulphonation or phosphoramide will reduce the antimicrobial activity o compound to zero.
- Increasing the crowd over beta-lactam ring increases the activity of the compound.
- By introducing more hydrophilic west-ends, the antimicrobial spectrum of the drug can be increased.
- Strong acidic groups at the alpha-carbonyl centre can also increase he spectrum of activity.
- Substitution of the sulphur with other groups decreases the antimicrobial activity of the compound.
- Penicillin activity is also highly dependent on the germinal dimethyl group at C-2.
- The reactivity of the beta-lactam ring is due to the presence of the nitrogen atom. Therefore, nitrogen atom is necessary for the antimicrobial activity of the compound.
- Doubly activated penicillin esters will undergo cleavae antibiotics to generate the active penicillin.
- Presence of urea groups will increase the penetration power of the compound, thus, it will be more active. [3]
METHOD OF SYNTHESIS
Penicillin is obtained from Penicillium. Penicillium produces penicillin when stress is applied on it by providing the condition which are not suitable for its growth.
Biosynthesis of penicillin follows the following pathway:
- L-valine undergo epimerization to produce D-valine.
- Condensation of L-α-aminoadipic acid, L-cysteine and D-valine, to give a tripeptide named, δ-(L-α-aminoadipyl)-L-cysteine-D-valine (ACV).
- Oxidation of ACV into isopenicillin N by the application of isopenicillin N synthase.
- Pehenylacetyl chain is replaced with α-aminoadipyl side-chain of isopenicillin N through transamidation with the application of α-aminoadipyl side-chain of isopenicillin N.
THERAPEUTIC USES: penicillin g is a narrow spectrum antibiotic. It is only effective against the gram positive bacteria. Penicillin is commonly given for the treatment of-
- Gingivostomatitis
- Pulmonary infections
- Genital disease
- Syphilis
- Meningococcal disease
- Pneumococcal infection
- Actinomycosis
- Anthrax
- Infective Arthritis caused by gonorrhea
- acute otitis media
- bacterial pharyngitis
SIDE EFFECTS
- Adverse drug reaction may cause- diarrhea, hypersensitivity, nausea, rashes and neurotoxicity.
- Infrequent side effects are– fever, vomiting, dermatitis, seizures and angioedema.
- Other side effects are– serum sickness, anaphylactic reactions in body, etc.
MULTIPLE CHOICE QUESTIONS
(A)What is the correct IUPAC nomenclature for ‘penicillin G’?
- (2S,5R,6R)-3,3-dimethyl-7-oxo-6-[(2-phenylacetyl)amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-ethanol.
- (2S,5R,6R)-3,3-dimethyl-7-oxo-6-[(2-phenylacetyl)amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid.
- (2S,5R,6R)-3,3-dimethyl-7-oxo-6-[(2-phenylacetyl)amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-sulphonic acid.
- (2S,5R,6R)-3,3-dimethyl-7-oxo-6-[(2-phenylacetyl)amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-acety chloride.
(B). Which type/s of ring/s are present in the Penicillin?
- Pyrolle ring
- Imidazol ring and thiazolidine ring
- β-lactam ring and thiazolidine ring
- β-lactam ring and pyrolle ring
(C). Penicillin is obtained from
- bacteria
- fungi
- virus
- protozoa
(D) Penicillin is effective for
- gram positive bacteria
- gram negative bacteria
- both gram positive and gram negative bacteria
- acid-fast bacteria
(E) Which statement/s is/are correct?
I. Penicillin is effective on gram negative bacteria
II. Beta lactam ring of penicillin binds with DD-transpeptidase of bacteria
III. Penicillin g is obtained from a fungi.
- I,II and III are correct
- II and III are correct
- II is incorrect
- Only I is correct
(F). The reactivity of the Beta-lactam ring of penicillin dependent on
- Nitrogen atom
- Hydrogen atom
- Carbon atom
- Oxygen atom
(G). Oxidation of δ-(L-α-aminoadipyl)-L-cysteine-D-valine (ACV) tripeptide gives
- Penicillin
- Amoxicillin
- Isopenicillin
- L-alanine
ANSWERS
(A). (2) (2S,5R,6R)-3,3-dimethyl-7-oxo-6-[(2-phenylacetyl)amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid.
(B). (3) β-lactam ring and thiazolidine ring, fused together.
(C). (2) pencillin is obtained by fungi penicillium
(D). (1) penicillin is only effective for gram positive bacteria
(E). (2) penicillin is only effective for gram positive bacteria
(F). (1) activity of beta-lactam ring is dependent on nitrogen atom
(G). (3) isopeniciilin is formed by oxidation of ACV tripeptide
REFERENCES
[1] Svenstrup N, Becher J. The organic chemistry of 1, 3-dithiole-2-thione-4, 5-dithiolate (DMIT). Synthesis. 1995 Mar;1995(03): pp.215-35. [2] Tripathi KD. Essentials of medical pharmacology. JP Medical Ltd; 2013 Sep 30.: pp. 297-298. [3] Wilson CO, Beale JM, Block JH. Wilson and Gisvold’s textbook of organic medicinal and pharmaceutical chemistry. Baltimore, MD: Lippincott Williams & Wilkins,; 2011: pp.271-272.