DUTASTERIDE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
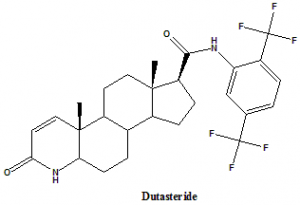
Dutasteride
IUPAC nomenclature
(1S,3aS,3bS,5aR,9aR,9bS,11aS)-N-[2,5-bis(trifluoromethyl)phenyl]-9a,11a-dimethyl-7-oxo-1,2,3,3a,3b,4,5,5a,6,9b,10,11-dodecahydroindeno[5,4-f]quinoline-1-carboxamide.
Classification
Dutasteride is a 5-α reductase inhibitor. [1]
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 528.5 g/mol |
| 2 | Appearance | Solid |
| 3 | Melting point | 242-250°C |
| 4 | Solubility | Insoluble |
| 5 | Octanol/water partition coefficient | 6.8 |
| 6 | Presence of ring | Quinoline ring |
| 7 | Number of chiral centers | 7 |
Mechanism of Action
i. Formation of stable complex with both type I and type II 5α-reductase, the conversion of testosterone into 5α-dihydrotestosterone is inhibited.
ii. Reducing the serum DHT level, there is reduction in the volume of prostate and epithelial apoptosis increases. [2]
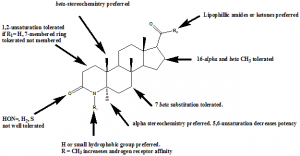
Structure Activity Relationship
Structure activity relationship of 5α- inhibitors can be better understood by the following representation:
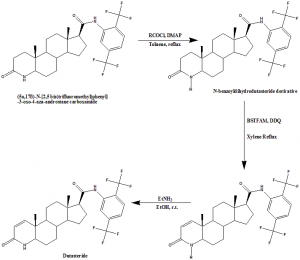
Method of synthesis
i. (5α,17β)-N-[2,5 bis(trifluoromethyl)phenyl]-3-oxo-4-aza-androstane carboxamide was treated with benzoyl chloride in presence of 4-dimethylaminopyridine to give N-benzoyldihydrodutasteride derivative.
ii. Silylation of amide functionality of N-benzoyldihysrodutasteride using N,O-bis(trimethylsilyl)-trifluoroacetamide to give enol, which favors C-2 carbon of the silylated compound to react with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone to give intermediate complex.
iii. Intermediate complex decomposed to due thermal treatment lead to dehydrogenation and thus gave N-benzoyl dutasteride derivatives.
iv. Hydrolysis of benzoyl group with hydrazine gave dutasteride in good yields.
Therapeutic Uses
The drug used for the treatment of:
- Benign prostate hyperplasia (enlarged prostate)
- Scalp hair loss
- Prostate cancer
- Hirsutism
- Transgender hormone therapy
Side Effects
Headache and GIT discomfort are adverse effects of this drug.
MCQs
Q.1 Trade name for the drug Dutasteride is?
a) Avodart
b) Mustine
c) Alkeran
d) Dacarin
Q.2 Predict the incorrect statement related to drug Dutasteride-
a) Have melting point 250 degree centigrade
b) Have 7 chiral carbon atoms
c) Used for the treatment of Scalp hair loss
d) None of the above
Q.3 Match the following drugs with the number of chiral centers they are having.
| i. Flutamide | A.1 |
| ii.Dutasteride | B. 9 |
| iii. Nafarelin | C. 7 |
| iv. Bicalutamide | D.0 |
a) i-A, ii-D, iii-B, iv-C
b) i-C, ii-A, iii-B, iv-D
c) i-D, ii-C, iii-B, iv-A
d) i-B, ii-D, iii-C, iv-A
Q.4 Which amongst the following drugs shows its effect through inhibition of 5-α reductase?
a) Finasteride
b) Dutasteride
c) Letrozole
d) Both a) and b)
Q.5 Dutasteride drug belongs to which class?
a) 5-α reductase inhibitor
b) GnRH analogue
c) Progestin
d) Aomatase inhibitor
Q.6 Dutasteride is used for the treatment of?
a) Benign Prostate hyperplasia
b) Scalp hair loss
c) Hirsutism
d) All of the above
Q.7 Quinoline ring is present in the structure of which of the following drug?
a) Finasteride
b) Dutasteride
c) Busulfan
d) Both a) and b)
ANSWERS
1-a
2-d
3-c
4-d
5-a
6-d
7-d
REFERENCES
[1] Tripathi KD. Essentials of Medical Pharmacology, 6thEdn. Jaypee Brothers Medical Publishers (P) Ltd. 2008: 820. [2] Lazier CB, Thomas LN, Douglas RC, Vessey JP, Rittmaster RS. Dutasteride, the dual 5α–reductase inhibitor, inhibits androgen action and promotes cell death in the LNCaP prostate cancer cell line. The Prostate. 2004 Feb 1;58(2):130-44.