TIOTROPIUM Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
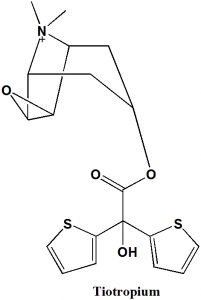
Tiotropium
IUPAC nomenclature
7-[(hydroxidi-2-thienylacetyl)oxy]-9,9-dimethyl-3-oxa-9-azoniatricyclo[3.3.1.02,4]nonane bromide
Classification
Tiotropium is a long-acting, antimuscarinic bronchodilator.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 392.5 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | 218-220°C. |
| 4 | Solubility | Feely soluble in dimethyl sulfoxide; sparingly soluble in water. |
| 5 | Presence of ring | Present |
| 6 | Number of chiral centers | 5 |
Mechanism of Action
- Tiotropium produces antagonist effect for muscarinic receptors from M1 to M5.
- It inhibits the M3 receptors present in the smooth muscle of the lung to produce relaxation and thus, bronchodilation. [1]
Structure Activity Relationship
- Either R1 or R2 must be heterocyclic or carbocyclic.
- The R3 group can be hydrogen, hydroxyl, hydroxymethyl or amide.
- Most potent derivatives has X as an ester.
- X can also be either oxygen or absent completely.
- The N substituent can be quaternary ammonium salt or tertiary amine or both with different alkyl groups.
- Maximum potency obtained when the distance between the ring substituted carbons is 2 carbon units.
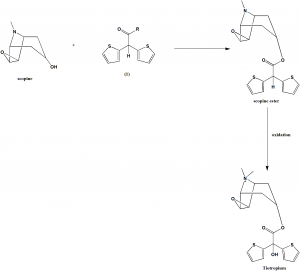
Method of synthesis
i. Scopine reacts with compoud with formula (1) to give scopine ester.
ii. Scopine ester undergoes oxidation to give Tiotropium.
Therapeutic Uses
Tiotropium is used for:
- Treatment of asthma
- Treatment of bronchitis
- Treatment of emphysema
- Prevention of wheezing
- Prevention of shortness of breath
- Controlling symptoms of other breathing problems
Side Effects
Side effects of Tiotropium are:
- Dry mouth
- Dizziness
- Allergic reactions
MCQ
Q.1 Tiotropium?
a) Stimulate nicotinic receptors
b) Stimulates muscarinic receptors
c) Inhibits nicotinic receptors
d) Inhibits muscarinic receptors
Q.2 Therapeutic use of drug tiotropium is/are?
a) Asthma
b) Bronchitis
c) Emphysema
d) All of the above
Q.3 Which amongst the following are the correct statements with respect to the SAR of drug Tiotropium?
I. Either R1 or R2 must be heterocyclic or carbocyclic.
II. The R3 group can be hydrogen, hydroxyl, hydroxymethyl or amide.
III. Most potent derivatives has X as an ester.
a) II
b) I
c) II, I
d) I, II, III
Q.4 The starting chemicals required for the synthesis of drug Tiotropium?
a) 2-(2’,2’-dimethoxyethyl)propane-1,3-diol.
b) Epoxypropane
c) Cyclobutane
d) Scopine
Q.5 Correct sequence for the True/False for the physiochemical properties of the drug Tiotropium?
- Imidazole ring is present
- Freely Soluble in water
- Present in oil or crystal form
a) TTT
b) TTF
c) FFT
d)FTF
Q.6 Correct statements for the IUPAC nomenclatures of the are?
I. Pilocarpine: (3S,4R)-3-Ethyl-4-((1-methyl-1H-imidazol-5-yl)methyl)dihydrofuran-2(3H)-one.
II. Orphinendrine: N,N-dimethyl-2-[(2-methylphenyl)-phenylmethoxy]ethanamine;2-hydroxypropane-1,2,3-tricarboxylic acid.
III. Tiotropium: 7-[(hydroxidi-2-thienylacetyl)oxy]-9,9-dimethyl-3-oxa-9-azoniatricyclo[3.3.1.02,4]nonane bromide
IV. Carbachol: 2-[(Aminocarbonyl)oxy]-N,N,N-trimethylethanaminium chloride.
a) I, III
b) II, IV
c) I, II, III, IV
d) I, II, IV
Q.7 Match the following drugs with their correct classifications-
| i. Pilocarpine | A. Acetylcholinestrase inhibitor |
| ii. Physostigmine | B. Acetylcholine antagonist-muscarinic antagonist |
| iii. Tiotropium | C. Acetylcholine mimetic-muscarinic agonist |
| iv. Metocurine | D. Nicotinic antagonist |
a) i-C, ii-A, iii-B, iv-D
b) i-B, ii-D, iii-A, iv-C
c) i-A, ii-B, iii-D, iv-C
d) i-B, ii-C, iii-D, iv-A
FREE GPAT online Test: Participate: Click Here
ANSWERS
1-d
2-d
3-d
4-d
5-d
6-c
7-a
REFERENCES
[1] Bateman ED, Rennard S, Barnes PJ, Dicpinigaitis PV, Gosens R, Gross NJ, Nadel JA, Pfeifer M, Racké K, Rabe KF, Rubin BK. Alternative mechanisms for tiotropium. Pulmonary pharmacology & therapeutics. 2009 Dec 1;22(6):533-42.