LORAZEPAM Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
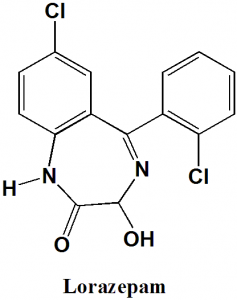
Lorazepam
IUPAC nomenclature
7-Chloro-5-(2-chlorophenyl)-3-hydroxy-1,3-dihydro-1,4-benzodiazepin-2-one
Classification
Lorazepam is a benzodiazepine sedative-hypnotic.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 321.2 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | 167°C |
| 4 | Octanol/water partition coefficient | 2.39 |
| 5 | Solubility | 80 mg/ml |
| 6 | Presence of ring | Diazepine, benzene |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
i. Lorazepam binds allosterically with the benzodiazepine receptor present in the post synaptic GABAA at different sites in CNS.
ii. This results in increases of the GABA inhibitory effects.
iii. Flow of chloride ions into the cell increases and hyperpolarization of cell takes place.
iv. Lorazeam binds in the amygdale to help in anxiety, while binding of the drug in cerebral cortex helps in seizure disorders.
Structure Activity Relationship
- Ring A should include an aromatic or heteroaromatic ring for binding with 5-phenyl-1,4-benzodiazepin-2-one derivatives.
- An electronegative group at 7-position of the ring A increases the functional anxiolytic activity.
- Substitutions at 6, 8 or 9 position with electronegative group on ring A will decrease the functional anxiolytic activity.
- When Heterocycles used as ring A, drug shows poor pharmacological activity.
- A proton-accepting group is essential on Ring B for binding with GABAA
- When the proton accepting group is present on the 2-position of the ring B, and is in coplanar spatial orientation with Ring A, maximum activity is observed.
- Replacement of oxygen with sulfur in ring B results in alteration in the selectivity for binding with GABA BZR subpopulations, but anxiolytic properties are maintained.
- There is no effect on agonist activity, but the antagonist activity dereases when methylene 3-position or imine nitrogen of the ring B is substituted.
- Derivatives having the 3-hydroxy moiety are fast excreted.
- Sterically large substituents on ring B , like tert-butyl group reduces the receptor affinity and the in vivo activity.
- 4,5-double bond and 4-position nitrogen is not essential for anxiolystic activity.
- BZR affinity is decreased if C=N bond is replaced with C-N bond.
- 5-phenyl ring C is not necessary for the binding with BZR.
- Substitution at the para position of the ring C decreases the agonist activity of the drug.
- There is no change observed in the agonist property of the drug when there is substitution at ortho position.
- When 1,2-bond f the ring C is annelated with an additional electron rich ring such as imidazole, affinity of the BZR increases. [1]
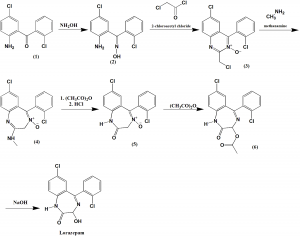
Method of synthesis
i. Reaction of 2-amino-2’5-dichlorobenzophenone (1) with hydroxylamine gives (2).
ii. (2) on reaction with chloroacetyl chloride and fter heterocyclization gives 6-chloro-2-chlormethyl-4-(2’-chlorphenyl)quinazolin-3-oxide (3)
iii. On reaction of (3) with methylamine leads to thering expansion and formation of 7-chloro-2-methylamino-5-(2’-chlorphenyl)-3H-1,4-benzodiazepin-4-oxide (4).
iv. On aetylation on secondary nitrogen using acetic anhydride, followed by hydrolysis by hydrochloric acid give 7-chloro-5-(2’-chlorphenyl)-1,2-dihydro-3H-1,4-benzodiazepin-2-on-4-oxide (5).
v. On further reaction with acetic anhydride, the compound will undergo Polonovski type rearrangement reaction to produce 3-acetoxylated benzodiazepine, 7-chloro-1,3-dihydro-3-acetoxy-5-(2′-chlorphenyl)-2H-benzodiazepin-2-one (6).
vi. Hydrolysis of (6) will yield lorazepam. [2]
Therapeutic Uses
Lorazepam is used for:
- Treatment of anxiety
- Treatment of seizures
- Producing sedation
Side Effects
Side effects of Lorazepam are:
- Dizziness
- Headache
- Nausea
- Loss of coordination
- Change in libido
- Loss/gain in appetite
- Heartburn
- Constipation
- Hallucinations
- Depression
- Suicidal thoughts
- Vision changes
- Weakness
- Memory loss
- Infections
- Sore throat
MCQ
Q.1 Correct statements related to the physicochemical properties of drug Lorazepam are?
I. Molecular weight: 299.75 gm/mol
II. Present in solid form at NTP.
III. Octanol/water partition coefficient is 4.4
IV. No chiral carbon atoms are present in structure
a) I, II
b) I II, III
c) II, IV
d) II, IV
Q.2 Match the following of the drugs with their correct IUPAC names.
| i. Flavoxate | A. 2-(1-piperidyl)ethyl 3-methyl-4-oxo-2-phenylchromene-8-carboxylate |
| ii. Eszopiclone | B. 7-Chloro-5-(2-chlorophenyl)-3-hydroxy-1,3-dihydro-1,4-benzodiazepin-2-one |
| iii. Chlordiazepoxide | C. 7-Chloro-2-methylamino-5-phenyl-3H-1,4-benzodiazepine-4-oxide |
| iv. Lorazepam | D. (S)-(+)-6-(5-Chloro-2-pyridinyl)-7-oxo-6,7-dihydro-5H-pyrrolo[3,4-b]pyrazin-5-yl-4-methyl-1-piperazinecarboxylate |
a) i-B, ii-C, iii-A, iv-D
b) i-D, ii-A, iii-B, iv-C
c) i-A, ii-B, iii-C, iv-D
d) i-A, ii-D, iii-C, iv-B
Q.3 Correct steps for the mechanism of action of the drug Lorazepam?
I. Binding with GABAA receptors
II. Increase the Binding of GABA to the GABAA receptors
III. Decreases of the binding of GABA to the GABAA receptors
IV.Enhancement of the GABA mediated chloride influx
a) I – III – IV
b) I – IV – II
c) II – III – IV
d) I – II – IV
Q.4 Correct sequence for True/false for the classification of the drug can be?
- Flavoxate: Nicotinic acetylcholine antagonist
- Aszopiclone: nonbenzodiazepine sedative-hypnotic
- Chlordiazepoxide: Muscarinic acetylcholine antagonist
- Lorazepam: Benzodiazepine sedative hypnotic
a) TTFT
b) FFTT
c) FTFT
d) TFTT
Q.5 When there is substitution at the ortho position of the ring C of Lorazepam-
a) No change observed in agonist property
b) Agonist property increases by 2-fold
c) Agonist property decreases by 2-fold
d) Agonist property increases by !.5-fold
Q.6 Types of ring structure present in Lorazepam ?
I. Chromine
II. Benzene
III. Piperidine
IV. Diazepine
a) I, II, III, IV
b) II, IV
c) I, III
d) I, II, III
Q.7 Side effect of drug Lorazepam?
a) Dizziness
b) Constipation
c) Blurred vision
d) All of the above
Participate in Free Online Test for GPAT, Pharmacist,Drug Inspector
ANSWERS
1-d
2-d
3-d
4-c
5-a
6-b
7-d