RITONAVIR Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Ritonavir
IUPAC nomenclature
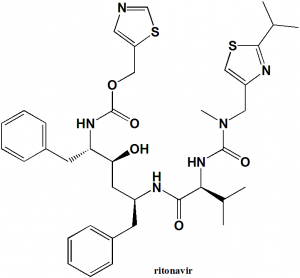
1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[(2S)-3-methyl-2-{[methyl({[2-(propan-2-yl)-1,3-thiazol-4-yl]methyl})carbamoyl]amino}butanamido]-1,6-diphenylhexan-2-yl]carbamate
Classification
Ritonavir is an HIV protease inhibitor.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 720.9g/mol |
| 2 | Physical appearance | White to tan powder |
| 3 | Melting point | 120-122°C |
| 4 | Solubility | Practically insoluble in water; freely soluble in methanol. |
| 5 | Octanol/water partition coefficient | 3.9 |
| 6 | Presence of ring | Thiazole, benzene |
| 7 | Number of chiral centers | 4 |
Mechanism of Action
- Ritonavir inhibits the HIV viral protienase enzymes which are involved in cleaving of the structural and replicative proteins which arise from Gag and Pol genes.
- Gag encodes for the proteins of the core and neucleocapsid.
- Pol encodes for HIV reverse transcriptase, ribonuclease H, protease and integrase.
- Gag-pol is the larger precursor which is initially translated and needed to be cleaved by HIV protease enzyme to develop other compliment proteins.
- Ritonavir inhibits the cleaving of gag-pol polyprotien, and thus, the immature and non-infectious viral particles are produced.
- Ritonavir is a type II ligand and it can potentially inhibits the cytochromae P450 CYP34A isoenzyme present in the instestinal tracts and liver. Drug fits into the active site of CYP34A and irreversibly binds with the heme iron through the thiazole nitrogen. This reduces the redox potential of the protein and precludes its reduction with redox partner, cytochrome P450 reductase.
- Ritonavir also limits the cellular transport and efflux of the other protease inhibitors through the P-glycoprotien and MRP efflux channels.
Structure Activity Relationship
- Replacement of the side chain with the conformationally constrained hexahydrofurofuranyloxy P(2) ligand in combination with a dimethylphenoxyacetate on the other end of the ritonavir core diamine yielded highly potent HIV protease inhibitors.
- Substituted groups on the P1 aromatic rings have influence on their biological activity.
- Compounds having an alkyl or fluorine atom at the meta or para position on their P1 benzene ring are good inhibitors.
- Substitutions on the P2 ring is also important for good antiviral property [1]
Method of synthesis
i. Valine condensed with bis-trichloromethyl carbonate to give an intermediate compound 4-isopropyloxazolidine-2,5-dione.
ii. The intermediate compound is reacted with compound (1) to give compound (2).
iii. (2) reacts with bis-trichloromethyl carbonate followed by reaction with (2-isopropylthiazol-4-yl)-N-methylmethanamine to give compound (3).
iv. Primary amine group of (3) is subjected to deprotection by the removal of two benzyl groups to give compound (4).
v. (4) reacts with (5) to give Ritonavir in good yield.
Therapeutic Uses
- Lopinavir and Ritonavir are given in combination for the treatment and prevention of HIV infection.
- Lopinavir and Ritonavir are given in combination for the treatment and prevention Novel Corona Virus Infection (COVID-19)
Side Effects
Side effects of Ritonavir are:
- Nausea
- Vomiting
- Diarrhea
- Headache
- Heartburn
- Stomach pain
- Tiredness
- Weakness
- Change in taste
- Numbness of mouth
MCQs
Q.1 What can be the correct Brand name oF drug ritonavir?
a) Norvir
b) Myleran
c) Busuphan
d) Endoxan
Q.2 Which amongst the following statements is/are incorrect related to the SAR of ritonavir?
I. Replacement of the side chain with the conformationally constrained hexahydrofurofuranyloxy P(2) ligand in combination with a dimethylphenoxyacetate on the other end of the ritonavir core diamine yielded highly potent HIV protease inhibitors.
II. Substituted groups on the P1 aromatic rings have influence on their biological activity.
III. Compounds having an alkyl or fluorine atom at the meta or para positionon their P1 benzene ring are poor inhibitors.
IV. Substitutions on the P2 ring is also important for good antiviral property
a) I
b) II
c) III
d) IV
Q.3 Type of rings present in the structure of ritonavir??
I. Thiazole
II. Pyrimidine
III. Benzene
IV. Cyclohexane
a) I, III
b) II, IV
c) III, IV
d) II
Q.4 Side effects of drug ritonavir is/are?
a) Headache
b) Weakness
c) Diarrhea
d) All of the above
Q.5 Match the following drugs with their correct molecular weights-
| i. Ritonavir | A. 305.2 gm/mol |
| ii. Melphalan | B. 156.05 gm/mol |
| iii. Cyclophosphamide | C. 261.08 gm/mol |
| iv. Mechlorethamine | D. 720.9 gm/mol |
a) i-C, ii-B, iii-D, iv-A
b) i-A, ii-B, iii-C, iv-D
c) i-B, ii-D, iii-C, iv-A
d) i-D, ii-A, iii-C, iv-B
Q.6 An example of drug from class HIV protease inhibitor?
a) Melphalan
b) Ritonavir
c) Clobazam
d) Pentobarbital
Q.7 Number of chiral centers present in the structure of ritonavir is?
a) 1
b) 2
c) 3
d) 4
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-a
2-c
3-d
4-b
5-d
6-b
7-d