AMPRENAVIR Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Amprenavir
IUPAC nomenclature
(3S)-oxolan-3-yl N-[(2S,3R)-3-hydroxy-4-[N-(2-methylpropyl)(4-aminobenzene)sulfonamido]-1-phenylbutan-2-yl]carbamate
Classification
Amprenavir is an HIV protease inhibitor.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 505.6 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | °C |
| 4 | Solubility | 40 mg/L in water |
| 5 | Octanol/water partition coefficient | log Kow = 2.2 at 25oC |
| 6 | Presence of ring | Furan, phenyl |
| 7 | Number of chiral centers | 3 |
Mechanism of Action
i. Inhibition of HIV viral proteinase enzyme by amprenavir.
ii. Noninfectious, immature viral particles are produced.
Structure Activity Relationship
- The receptors are bean shaped having a predominantly hydrophobic pocket in the upper half of the region with distinct ‘V’ shape.
- The hydrophilic region is present at the periphery having H-bond acceptor rich area which can also accommodate a positive salt bridge in the form of terminal aromatic ring.
- The bean shape structure of the inhibitor has a prominent H-bond acceptor region lies in the center depression.
- In the ionic or hydrophilic sites, ligands may form H-bonds with the active site of the receptor.
- The absolute planarity in the ligand structure is necessary for the pi-pi –interactions with the receptor. [1]
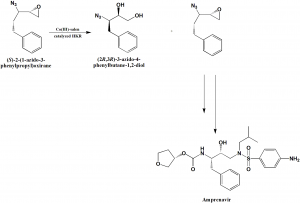
Method of synthesis
Co-catalyzed stereo centered hydrolytic kinetic resolution of racemic 2-(1-azido-2-phenylethyl)oxirane as the chirality inducing step. There is also synthesis of (S)-3-hydroxytetrahydrofuran with 98% enantiomeric property.[2]
Therapeutic Uses
- Amprenavir for the treatment and prevention of HIV infection.
Side Effects
Side effects of Amprenavir are:
- Nausea
- Vomiting
- Diarrhea
- Abdominal pain
MCQs
Q.1 What can be the brand name for drug Amprenavir?
a) Agenerase
b) Anethane
c) Xylocaine
d) Tricaine
Q.2 Which amongst the following statements is/are incorrect related to the SAR of amprenavir?
I. The receptors are bean shaped having a predominantly hydrophobic pocket in the upper half of the region with distinct ‘V’ shape.
II. The hydrophilic region is present at the periphery having H-bond acceptor rich area which can also accommodate a positive salt bridge in the form of terminal aromatic ring.
III. The bean shape structure of the inhibitor has a prominent H-bond acceptor region lies in the center depression.
IV. In the iomic or hydrophilic sites, ligands may form H-bonds with the active site of the receptor.
a) I, II
b) II, IV
c) III
d) None
Q.3 Type of ring present in the structure of Amprenavir?
I. Phenyl
II. Cyclopentane
III. Pyrimidine
IV. Furan
a) I, III
b) II, III
c) I, IV
d) II, IV
Q.4 Side effects of drug Amprenavir is/are?
a) Nausea
b) Vomitting
c) Diarrhea
d) All of the above
Q.5 Match the following drugs with their correct molecular weights-
| i. Amprenavir | A. 226.27 gm/mol |
| ii. Amobarbital | B. 294.27 gm/mol |
| iii. Estazolam | C. 505.6 gm/mol |
| iv. Quazepam | D. 386.6 gm/mol |
a) i-C, ii-A, iii-B, iv-D
b) i-D, ii-B, iii-C, iv-A
c) i-B, ii-A, iii-D, iv-C
d) i-A, ii-B, iii-C, iv-D
Q.6 An example of drug from class HIV protease inhibitor is?
a) Cytarabine
b) Methotraxate
c) Amprenavir
d) 5-FU
Q.7 Number of chiral centers present in the structure of Amprenavir?
a) 0
b) 1
c) 2
d) 3
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-a
2-d
3-c
4-d
5-a
6-c
7-d