BUMETANIDE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Bumetanide
IUPAC nomenclature
3-butylamino-4-phenoxy-5-sulfamoyl-benzoic acid
Classification
- Sulfamyl
- Diuretics
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 364.4 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | 230.5oC |
| 4 | Solubility | >20 mg/ml |
| 5 | Octanol/water partition coefficient | 2.6 |
| 5 | Presence of ring | Phenyl |
| 6 | Number of chiral centers | Not present |
Mechanism of Action
- Bumetanide inhibits the sodium-potassium ATPase pump and interferes with renal cAMP.
- It blocks the active reabsorbtion of sodium and chloride in the ascending loop of Henle, and also alters the electrolyte transfer in PCT, thereby; there is increase in the excretion of sodium, chloride and water from the body.
Structure Activity Relationship
General structure activity relationship of bumetanide can be summarized as:.
- There is replacement of chloro and trifluoromethyl groups as in other diuretics with phenoxy group.
- Amine group is also shifted from 6th position to 5th
- Replacement of phenoxy group with C6H5NH- or with C6H5S- groups also gives compounds with favorable activity.
- Replacement of butyl group at C-5 position with a furanylmethyl group, compound so obtained has unfavorable activity. [1]
Method of synthesis
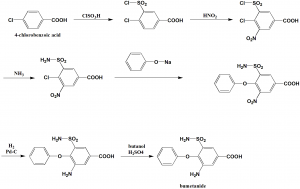
i. 4-chlorobenzoic acid undergoes sulfonylchlorination by the help of chlorosulfonic acid to get 4-chloro-3-chlorosulfonylbenzoic acid.
ii. Nitration of the above formed compound using nitric acid to get the compound 4-chloro-3-chlorosulfonyl-5-nitrobenzoic acid.
iii. On reacting the last with sodium phenolate, 5-amino-sulfonyl-3-nitro-5-phenoxybenzoic acid is produced.
iv. Reducing the nitro group of above formed product by using hydrogen using a palladium on carbon catalyst produces the compound 3-amino-5-aminosulfonyl-5-phenoxybenzoic acid.
v. Reacting the last with butyl alcohol in presence of sulfuric acid gives the drug bumetanide. [2]
Medicinal Uses
Bumetanide is used for:
- Treatment of edema related with CHF, liver cirrhosis, renal diseases.
Side Effects
Side effects of Bumetanide are:
- Dizziness
- Lightheadedness
- Dehydration
- Muscle cramps
- Weakness
- Fatigue
- Confusion
- Fainting
- Drowsiness
- Dry mouth
- Nausea
- Vomiting
- Irregular heartbeat
- Allergic reactions
MCQs
Q.1 Which of the following statements are correct related with the physicochemical properties of drug Hydrochlorothiazide?
I. Molecular weight: 364.4 gm/mol
II. Physical appearance: Solid
III. Melting point: 230.5 oC
a) I, II, III
b) I, II
c) II, III
d) I, III
Q.2 Match the following of the drugs with their correct IUPAC names.
| i. Hydrochlorothiazide | A. 4-(5H-Dibenzo[a,d]cyclohepten-5-ylidene)-1-methylpiperidine |
| ii. Bumetanide | B. 6-chloro-1,1-dioxo-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide |
| iii. Pantoprazole | C. 3-butylamino-4-phenoxy-5-sulfamoyl-benzoic acid |
| iv. Cyproheptidine | D. (RS)-6-(Difluoromethoxy)-2-[(3,4-dimethoxypyridin-2-yl)methylsulfinyl]-1H-benzo[d]imidazole
|
a) i-B, ii-C, iii-D, iv-A
b) i-D, ii-C, iii-B, iv-A
c) i-D, ii-B, iii-A, iv-C
d) i-B, ii-A, iii-D, iv-C
Q.3 Mechanism of action of drug Bumetanide includes?
I. Inhibition of sodium-potassium ATPase pump
II.Interferes with renal cAMP
III. Alters the electrolyte transfer in PCT
IV. Increase in excretion of water from body
a) I, III, IV
b) II, IV
c)I, II, III
d) I, II, III, IV
Q.4 Correct sequence for True/false for the classification of the drug can be?
- Bumetanide: Diuretics
- Tolazoline: α-adrenergic blocker
- Betazolol: ß-adrenergic blocker
- Naphazoline: Inhalational anesthetics
a) TFFT
b) FFTT
c) FFFF
d) TTTF
Q.5 Which of the following statements related with the SAR of Bumetanide is/are correct?
a) There is replacement of chloro and trifluoromethyl groups as in other diuretics with phenoxy group.
b) Amine group is also shifted from 6th position to 5th
c) Replacement of phenoxy group with C6H5NH- or with C6H5S- groups also gives compounds with favorable activity.
d) All of the above
Q.6 Type of rings present in the structure of Bumetanide?
I. Phenyl
II. Benzothiazine
III. Dihydrofuran
IV. Cyclobutane
a) II, IV
b) I
c) III, IV
d) II
Q.7 Side effect of drug Bumetanide is/are?
a) Dehydration
b) Muscle cramps
c) Fainting
d) All of the above
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
ANSWERS
1-a
2-a
3-d
4-d
5-d
6-b
7-d