PROPATYL NITRATE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
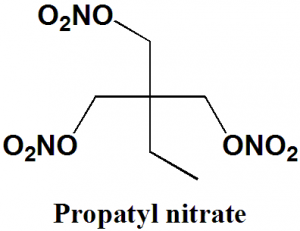
Propatyl Nitrate
IUPAC nomenclature
2,2-bis(nitrooxymethyl)butyl nitrate
Classification
- Organic nitrate antianginal drug
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 269.17 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | 51.5oC |
| 4 | Solubility | N/A |
| 5 | Octanol/water partition coefficient | N/A |
| 5 | Presence of ring | Not present |
| 6 | Number of chiral centers | Not present |
Mechanism of Action
i. Like other organic nitrates, propatyl nitrate releases NO.
ii. Activation of enzyme gunanylate cyclase
iii. Increase in concentration of cGMP within vascular smooth muscles
iv. Vasodilation occurs due to cGMP-dependent protein kinase.
Structure Activity Relationship
General structure activity of organic nitrate antianginal drugs be summarized as:
- The number of nitrate groups determines the potency of organic nitrate for guanylate cyclase activation.
- Increase in nitric group increases the potency.
- Increase in lipophillicity doesn’t have major effect over activation of drug.
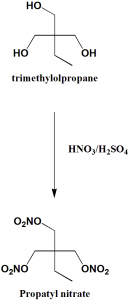
Method of synthesis
Nitration of trimethylolpropane produces Propatyl nitrate. [1]
Medicinal Uses
Propatyl nitrate is an antianginal drug
Side Effects
Side effects of Propatyl nitrate are:
- Dizziness
- Headache
- Flushing
- Nausea
- Vomiting
- Weakness
- Restlessness
- Allergic reactions
MCQs
Q.1 Which of the following statements are correct related with the physicochemical properties of drug Propatyl nitrate?
I. Molecular weight: 269.17 gm/mol
II. Physical appearance: Oil
III. Melting point: 51.5 oC
a) I, II, III
b) I, II
c) II, III
d) I, III
Q.2 Match the following of the drugs with their correct IUPAC names.
| i. Propatyl nitrate | A. 4-(5H-Dibenzo[a,d]cyclohepten-5-ylidene)-1-methylpiperidine |
| ii. Esomeprazole | B. 2,2-bis(nitrooxymethyl)butyl nitrate |
| iii. Hydroxyzene | C. (S)-(−)-5-Methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methylsulfinyl]-3H-benzoimidazole |
| iv. Cyproheptidine | D. (±)-2-(2-{4-[(4-chlorophenyl)-phenylmethyl]piperazin-1-yl}ethoxy)ethanol |
a) i-B, ii-C, iii-D, iv-A
b) i-D, ii-C, iii-B, iv-A
c) i-D, ii-B, iii-A, iv-C
d) i-B, ii-A, iii-D, iv-C
Q.3 Mechanism of action of drug Propatyl nitrate includes?
I. Release if NO
II. Activation of gunaylate cyclase enzyme
III. Active synthesis of cGMP
IV. Vasoconstriction
a) I, III, IV
b) I, IV
c)I, II, III
d) I, II, III, IV
Q.4 Correct sequence for True/false for the classification of the drug can be?
- Propatyl nitrate: Organic nitrate antianginal drug
- Bethanchol: α-adrenergic blocker
- Labetolol: ß-adrenergic blocker
- Chlorazepate: Inhalational anesthetics
- a) TFFT
b) FFTT
c) FFTF
d) TTTF
Q.5 Which of the following statements related with the SAR of dihydropyridines calcium channel blockers is/are correct?
a) The number of nitrate groups determines the potency of organic nitrate for guanylate cyclase activation.
b) Increase in nitric group increases the potency.
c) Increase in lipophillicity doesn’t have major effect over activation of drug.
d) All of the above
Q.6 Type of rings present in the structure of Propatyl nitrate?
I. Dihydropyridine
II. Phenyl
III. Dihydrofuran
IV. Cyclobutane
a) II, IV
b) I , II
c) III, IV
d) None of the above
Q.7 Side effect of drug propatyl nitrate is/are?
a) Weakness
b) Headache
c) Vomiting
d) All of the above
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
ANSWERS
1-d
2-a
3-c
4-d
5-d
6-d
7-d
REFERENCES
[1] Médard: Meml. Poudres (MPOUAT) 35, 113 (1953).