Colloidal Dispersion: Definition and Types and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam
A disperse system is defined as a heterogeneous two-phase system in which the disperse phase is distributed as particles or droplets throughout another phase called as dispersion medium. Based on the size of dispersed particles within the dispersion medium, the dispersed system can be classified as molecular dispersions, colloidal dispersions and coarse dispersions. Molecular dispersions are homogeneous in character and form true solutions. Dispersions in which the droplet size frequently exceeds 1 μm are referred to as coarse dispersions, which include most pharmaceutical suspensions and emulsions.
The term colloidal is usually applied to systems in which the size of the dispersed particles are intermediate in size between true solutions and coarse dispersions and are usually within the range 10–9 m (1 nm) to about 10–6 m (1 μm).
CLASSIFICATION OF COLLOIDS:
Based on the interaction between the particles of the dispersed phase and the molecules of the dispersion medium, colloidal systems are classified into three groups.
Lyophilic Colloids- Lyophilic colloids are solvent-loving colloids, in which the disperse phase is dissolved in the continuous phase. Proteins and gums form lyophilic colloidal systems because of the affinity between the dispersed particles and the continuous phase. Lyophilic colloidal solutions are thermodynamically stable and form spontaneously when a solute and a solvent are brought together. There is a reduction in Gibbs free energy (∆G) on dispersion of a lyophilic colloid. G is related to the interfacial area (∆A), the interfacial tension (ϒ) and the entropy of the system (∆S), as follows:
∆G = ϒ∆A – T
where T is the absolute temperature.
Examples of lyophilic colloids include gelatin, carbopol and chitosan, which form colloidal dispersions in water.
Lyophobic Colloids- Lyophobic colloids are solvent-hating colloids, in which the disperse phase is insoluble in the continuous phase. The disperse phase is broken down into very small particles, which are distributed more or less uniformly throughout the solvent. The disperse phase and the dispersion medium may consist of solids, liquids or gases and are two-phase or multiphase systems with a distinct interfacial region. As a consequence of the poor dispersed phase-dispersion interactions, lyophobic colloids are thermodynamically unstable and have a tendency to aggregate. When water is used as the dispersion medium in such types of colloid, they are known as hydrophobic colloids. The ∆G increases when a lyophobic material is dispersed throughout a medium. The greater the extent of dispersion, the greater the total surface area exposed and hence the greater the increase in the free energy of the system. When a particle is broken down into smaller particles, work is needed to separate the pieces against the forces of attraction between them (W). The resultant increase in free energy is proportional to the area of the new surface created (A):
G = W = 2ϒA
Examples of lyophobic colloidal dispersions include gold and silver dispersed in water.
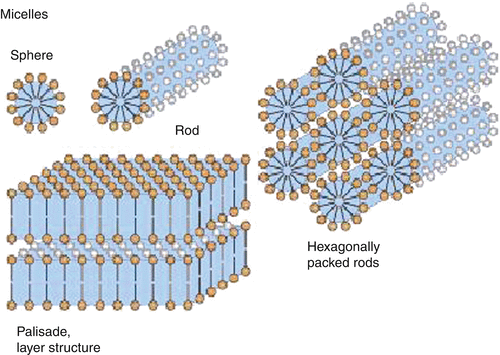
Association Colloids (Micelles)- Association colloids are systems in which the soluble amphipathic surfactant molecules spontaneously self-assemble or associate in the dispersion medium to form aggregates in the colloidal size range. Amphipathic surfactant molecules tend to adsorb at interfaces to reduce the interfacial energy between the lyophobic portion of the molecule and the medium. At concentrations above the critical micelle concentration, the lyophobic portions of surfactant molecules associate to form regions from which the solvent is excluded, whereas the lyophilic portions of the molecules remain on the outer surface. The micelle formation is spontaneous, depending on the HLB of the surfactant, concentration of the surfactant and the temperature. It appears that a bulky oxygen-containing hydrophilic group or a charged hydrophilic group is required to form micelles in an aqueous medium. These hydrophilic groups undergo significant hydrogen bonding and dipole interactions with water to stabilize the micelles.
- Micellar solubilization allows incorporation of water-insoluble drugs in aqueous medium.
- Entrapment of drug within a micellar system increases its stability and can enhance drug bioavailability.
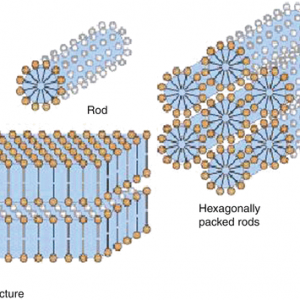
Fig 1 – Association colloids(taken from Colloidal state and its development Springer link)
Multiple choice questions(MCQs)
1.An example for colloidal system is
a)Gas and liquid
b)Gas and solid
c)Liquid and gas
d)Solid and gas
2.The criterion to call a system ‘colloid’ is
a)Electrolytes in small amounts induce stabilization
b)It is reversible
c)Particles have high electric charge
d)Viscosity increases by the presence of particles
3.Solutions of protein and starch in water are the examples of the colloidal type
a)Gas and water
b)Liquid and water
c)Solid and water
d)Water and solid
4.Electro dialysis method is employed in the colloidal chemistry for the purpose of
a)Amphiphiles
b)Colloids
c)Electrolytes
d)Nonelectrolytes
5.Which of the following do not form colloid spontaneously?
a)Bright specks against dark background
b)Concentric rings
c)Dark specks against bright background
d)Fluorescent specks
6.Sulphur solution is an example of colloid type
a)Measuring the change in particle size
b)Noting sedimentation volume of gold
c)Observing the color change
d)Weighing of the precipitate
7.Surfactant solutions are termed as association colloids when their concentrations are
a)Cellophane
b)Cellulose acetate
c)Polythene
d)Polyvinyl acetate
8.Name the type of colloidal dispersion to which electrolytes are normally added in small quantities to stabilize
a)Formation of micelles
b)Hydration of solids
c)Lowering interfacial tension
d)Presence of electrical charge
9.Protective colloids do not
a)Ultracentrifuge
b)Ultrafilters
c)Ultramicroscope
d)Zeta meter
10.Which of the following colloid is difficult to prepare?
a)Molecular dispersions
b)Colloidal dispersions
c)Suspension
d)Emulsion
11.In high concentrations, electrolytes destabilize a lyophilic solution by a process termed as
a)Electrophoresis
b)Electro-osmosis
c)Electrochemical reaction
d)Electro dialysis
12.Which quality of dispersed phase is responsible for the increased viscosity of a hydrophilic colloidal dispersion?
a)Streaming potential
b)Oxidation potential
c)Reduction potential
d)Sedimentation potential
13.Dispersion of acacia in water gives the colloid of type
a)Presence of valence or charge of the ions
b)Lowering the interfacial tension
c)Decreasing th freezing point
d)Elevation of boiling point
14.Addition of alcohol to a hydrophilic colloid leads to
a)Brownian movement
b)Diffusion
c)Tyndall effect
d)Donnan effect
15.The critical value of zeta potential(in milli volts)for a stable colloid(except gold sol)is
a)Brownian movement
b)Tyndall effect
c)Diffusion
d)Sedimentation
Solutions:
- a)Gas and liquid
- c)Particles have high electric charge
- c)Solid and water
- c) Electrolytes
- c)Bright specks against dark background
- c)Observing the color change
- a)Cellophane
- b)Hydration of solids
- a)Ultracentrifuge
- a)Molecular dispersions
- a)Electrophoresis
- d)Sedimentation potential
- a)Presence of valence or charge of the ions
- c)Tyndall effect
- a)Brownian movement
References:
1. GAURAV KUMAR JAIN – THEORY & PRACTICE OF PHYSICAL PHARMACY, 1st edition 2012 Elsevier, page no. 191-194.
2. Martins Physical Pharmacy, 6th edition 2011, page no. 710-715.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE