Solvents, Types of Solvents, Solvents for Pharmaceutical Use, and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam
TYPES OF SOLVENTS FOR PHARMACEUTICAL USE :
- PURIFIED WATER: It has a solvent effect on most substances, Purified water is water that is mechanically filtered or processed to be cleaned for consumption. Distilled water and deionized (DI) water have been the most common forms of purified water and it contains vary amounts of in- organic salts usually Na,K,Ca+2, iron, Mg, Cl , sulfate and bicarbonate. It is used in preparation of all medication containing water except non – parenteral solutions & some official external preparations such as liniments.
- ALCOHOL USP: SUCH AS ETHYL ALCOHOL, ETHANOL, C2H5OH: 1- Alcohol is next to water is the most useful solvent. 2-it is a good solvent for many organic subs. both natural &synthetic. 3-it is dissolve important plant constituents such as resins, volatile oils, alkaloids, glycosides &neutral principles. 4- Together with water it forms hydro-alcoholic solvent which dissolves both water soluble and alcohol soluble substance and to extract active constituents from crude drug. 5- Dehydrated alcohol USP (absolute alcohol) contains not less than (99.5%). 6-Alcohol has advantages over the water; it is not subjected to deterioration (growth of microorganisms). ((Used in liquid products as an antimicrobial preservative alone or with other agents)). 7- Alcohol is frequently used with other solvents such as glycols & glycerin to reduce the amount of alcohol required. 8-It is used for OTC oral products intended for children under (6 years), the recommended alcohol contains limit for product is over: (0.5%) for children under (6 years), (5%) for children (6-12 years), (10 %) for over 12 years.
- DILUTED ALCOHOL (NF): It is prepared by mixing equals volumes of alcohol USP with purified water USP. The final volume of such mixtures is not the sum of the individual volumes of the two components because the liquids contract upon mixing so the final volume is generally about 3% less than would be expected. Diluted alcohol is a useful hydro alcoholic solvent in various pharmaceutical processes and preparation.
- ISOPROPYL ALCOHOL: It is about 70% by volume Isopropyl alcohol, the remaining consisting of water with or without color additives ,stabilizers & perfume oils .it is used externally as a rubefacient & soothing rub & as a vehicle for topical products. This preparation is 91% isopropyl alcohol solution is commonly used by diabetic patients in preparing needles & syringes for subcutaneous injections of insulin & for disinfecting the skin. Also Used as solvent in cosmetic and dermatologic preparations.
- GLYCERIN USP (GLYCEROL): 1-It’s clear liquid with sweet taste. 2- It is miscible with both water and alcohol but not with ether and chloroform. 3- As a solvent it is viscous, therefore solute is slowly soluble in it, unless it is render to less viscosity by “heating”. 4-Glycerin has preservative stabilizer property. 5- It is used in many internally preparations (suppository) and ear drops because it softens the wax found in the ear.
- PROPYLENE GLYCOL USP: Viscous liquid miscible with water and alcohol in all proportion and is soluble in ether, acetone and chloroform. It is useful solvent with a wide range of applications and is frequently substituted with glycerin in modern pharmaceutical& cosmetic preparations.
- POLY ETHYLENE GLYCOL 400: 1-it is miscible with water, acetone, alcohol & other glycols. 2-it dissolves many water–soluble organic compounds & certain water soluble subs. Such as acetyl salicylic acid & theophylline.
- KETONE: There are only two official solvent-vehicles in the ketone group. 1. Acetone 2. Methyl iso Butyl Ketone Methyl ethyl ketone is not an official substance, But according to the section of USP it is used as a solvent for assays, tests and processing. Why we not use ketone officially? Officially ketone have limited usefulness because of their; Volatility, flammability and toxicity. They do have some unique solvent properties which make them useful. It is miscible with water, alcohol, ether, chloroform and in most of the oils. Precaution: It is stored in tight containers and stored remote from fire.
1.Water:
INN: Purified Water; Aqua purificata
Synonyms: Aqua; hydrogen oxide
Chemical Name & CAS Number: Purified Water (7732-18-5)
Molecular Formula: H2O
Description: Clear, colorless, odorless, and tasteless liquid.
Properties: Boiling point 100°C, freezing point 0°C; miscible with most polar solvents.
Incompatibilities: With drug subject to hydrolysis; reacts violently with alkali metals
Use: Most widely used excipient in pharmaceutical manufacturing; solvent for granulating agents; solvent for syrups, suspensions; vehicle for aqueous solutions; in oral solid and liquid processing; in parenteral processing as water for injection or sterile water for injection; in topical, ocular, otic delivery systems; to clean all processing equipment.
Special Classes of Water:
Purified Water: Obtained by distillation, ion-exchange treatment, reverse osmosis, or any other suitable process; contains no added substance.
Water for Injection: Purified by distillation or by reverse osmosis under aseptic conditions; not sterilized by filtration or in the final container.
Bacteriostatic Water for Injection: Sterile water for injection containing one or more suitable antimicrobial agents.
2.Glycerin:
INN: Glycerol, Glycerin
Synonyms: Glycerine, trihydroxypropane glycerol
Chemical Name & CAS Number: 1,2,3-Propanetriol (56-81-5)
Molecular formula: C3H8O3
Structure:
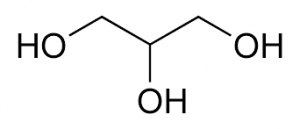
Description: Clear, colorless, syrupy liquid with a sweet taste and not more than a slight, characteristic odor, which is neither harsh nor disagreeable; when exposed to moist air, it absorbs water and such gases as H2S and SO2; solutions are neutral; specific gravity not less than 1.249 (not less than 95% C3H5(OH)3); boils at about 290°C under 1 atm, with decomposition, but can be distilled intact in a vacuum.
Properties: Miscible with water, alcohol; density 1.26 g/cc at room temperature; very hygroscopic.
Incompatibilities: An explosion may occur, if it is triturated with strong oxidizing agents, such as chromium trioxide, potassium chlorate, or potassium permanganate. In dilute solutions, the reactions proceed at a slower rate, forming several oxidation products. Iron is an occasional contaminant and may be the cause of a darkening in mixtures containing phenols, salicylates, tannin, etc. With boric acid or sodium borate, it forms a complex, spoken of as glyceroboric acid, which is a much stronger acid than boric acid.
Uses: For its humectant and emollient activity in topical formulations; as a solvent in parenteral formulations and a plasticizer for gelatin and other polymeric coatings.
3.Polyethylene glycols:
INN: Macrogol; Marcogolum; Polyethylene glycol
Synonyms: PEG; polyoxyethylene glycol
Chemical Name & CAS Number: α-Hydro-ωhydroxypoly(oxy-1,2-ethanediyl) (25322-68-3)
Molecular Formula: HOCH2(CH2OCH2)mCH2OH
Description: Polyethylene glycols 200, 300, 400, and 600 are clear, viscous liquids at room temperature. Polyethylene glycols 900, 1000, 1450, 3350, 4500, 6000, 8000 and 20000 are white, waxy solids.
Properties: All members of this class dissolve in water to form clear solutions and are soluble in many organic solvents. As their molecular weight increases, water solubility, vapor pressure, hygroscopicity, and solubility in organic solvents decrease, whereas freezing or melting range, specific gravity, flash point, and viscosity increase.
Incompatibilities: Some colors may react with these materials, due to the terminal hydroxyl groups; reduced penicillin activity occurs with these materials as is preservative effectiveness of parabens.
Uses: As a viscosifier for liquid products; a solubilizer for topical products; a vehicle for some parenteral products; an emollients and suppository bases in semi-solid products; a tablet/ capsule lubricant; a solubilizing agent for oral drug delivery.
Multiple choice questions:
1.Which of the following types of solvents are for pharmaceutical use?
a)Purified water
b)Alcohol
c)Glycerin
d)All of these
2.. Distilled water and deionized (DI) water have been the most common forms of purified water and it contains vary amounts of in- organic salts usually
a)Na
b)K
c)Ca+2
d)All of these
3.Alcohol can dissolve important plant constituents such as
a)resins
b)volatile oils
c)alkaloids
d)all of these
4.Which of the following is/are advantages of alcohol over the water?
a)it is not subjected to deterioration (growth of microorganisms)
b)it is easily available
c)it is cheap
d)all of these
5.Which of the following is prepared by mixing equals volumes of alcohol USP with purified water USP?
a)Alcohol
b)Diluted alcohol
c)Isopropyl alcohol
d)All of these
6.Isopropyl alcohol is used as
a)rubefacient
b)soothing rub
c)a vehicle for topical products
d)all of these
7.Isopropyl alcohol is used as solvent in cosmetic and dermatologic preparations.
a)true
b)false
8.Glycerin is used in
a)suppository
b)ear drops
c)both of these
d)none of these
9.Propylene glycol Viscous liquid miscible with water and alcohol in all proportion and is soluble in
a)ether
b)acetone
c)chloroform
d)all of these
10.PEG 400 is miscible with
a)water
b)acetone
c)alcohol
d)all of these
11.Which of the following are official solvent-vehicles in the ketone group?
a)Acetone
b)Methyl iso Butyl Ketone
c)Both of these
d)None of these
12.Hydrogen oxide is synonym of
a)Water
b)Glycerin
c)PEG
d)Acetone
13.Which of the following is obtained by distillation, ion-exchange treatment, reverse osmosis, or any other suitable process; contains no added substance?
a)Purified Water
b)Water for Injection
c)Bacteriostatic Water for Injection
d)All of these
14.C3H8O3 is formula of
a)Glycerin
b)PEG
c)Propylene glycol
d)IPA
15.Polyethylene glycol is used as
a) viscosifier for liquid products
b) a solubilizer for topical products
c)a vehicle for some parenteral products
d)all of these
Solutions:
- d)All of these
- d)All of these
- d)all of these
- a)it is not subjected to deterioration (growth of microorganisms)
- b)Diluted alcohol
- d)all of these
- a)true
- c)both of these
- d)all of these
- d)all of these
- c)Both of these
- a)Water
- a)Purified Water
- a)Glycerin
- d)all of these
References:
- Remington Essential of Pharmaceutics, 1st edition 2013, page no. 693, 698, 703.
- Raymond C Rowe Handbook of Pharmaceutical Excipients, 6th edition, page no. 283-286, 517-522, 766-770.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test