Absorption BASIC: Beer’s Law; Lambart’s Law and Equation
Absorption BASIC
First we take one example we have one radio source and we have one sample.
When our radiation source is emitting radiation it is called Incident radiation.
And Incident radiation have 100% intensity. And it is denoted by Io.
When incident radiation is passed through out sample it is called transmitted radiation.
And it is denoted by I.
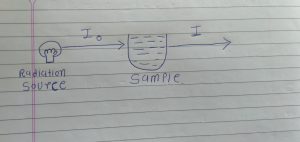
Above condition we have two possible situation.
Situation 1. If sample solution is absorbed radiation
Absorption is more than 0.
Transparent is less than 100%.
So, Io>I
∴A=Io/I
∴T = I/Io
∴ A= log10 (1/T)
T =I/Io so, 1/T = Io/I
A= log10 ( Io/I)
Situation 2 :-If sample solution is transparent.
A% is 0, T% is 100%
∴ Io= I
Beer’s Law :-
When a beam of monochromatic radiation is passed through the absorbing radiation then the decrease in the intensity of the radiation will be directly proportional to the concentration of the solution.
Equation for beer law is
A = ξ *C
Where,
A = absorption
ξ = molar extinction co-efficient
C = conc. of the solution
Lambart’s Law
When a beam of monochromatic radiation is passed through the absorbing medium then the decrease in the intensity of radiation will be directly proportional to the thickness of the solution.
Lamber’s Law Equation
A = ξ*L
Where
A = absorption
ξ = molar extinction co-efficient
L = path length
Beer – Lambert’s Law :-
When a beam of monochromatic radiation is passed through the absorbing medium then the decrease in the intensity of the radiation is directly proportional to the thickness of the medium and the concentration of the solution.
Equation :-
A = ξ*L*C
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE