ACTINOMYCIN D Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
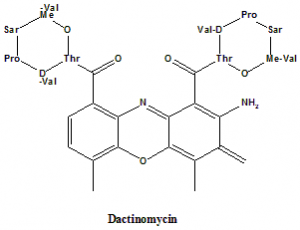
Actinomycin D (Dactinomycin)
IUPAC nomenclature
2-Amino-N,N′- bis[(6S,9R,10S,13R,18aS)-6,13-diisopropyl-2,5,9-trimethyl-1,4,7,11,14-pentaoxohexadecahydro-1H-pyrrolo[2,1-i][1,4,7,10,13]oxatetraazacyclohexadecin-10-yl]-4,6-dimethyl-3-oxo-3H-phenoxazine-1,9-dicarboxamide.
Classification
Actinomycin D falls under the category of Antibiotic cytotoxic drug. [1]
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 1255.4g/mol |
| 2 | Appearance | Bright red rhomboid prisms / red powder |
| 3 | Melting point | Range between 245-248°C |
| 4 | Solubility | 1 gm can be dissolved in 1000 ml of water |
| 5 | Octanol water partition coefficient | 3.21 |
| 6 | Presence of ring | Phenoxazone ring structure |
Mechanism of Action
i. Dactinomycin binds with DNA.
ii. RNA synthesis is prevented.
iii. Thus, protein synthesis will be disturbed. [2]
Structural Activity Relationship
- Lactone rings in the dactinomycin stabilizes the peptide chains in such a conformation which enables the formation of four extra Hydrogrn bonds between phosphodiester oxygens and peptide-NH groups.
- Amino acid composition contributes to the biological activity of dactinomycin.
- Replacing the proline by hydroxylproline can change the minimal inhibitory concentration of the drug.
- The different conformation in the peptide can result in the different biological activity of the drug.
- If pipecolic acid is attached with the drug then it can only inhibit the growth of gram positive bacteriabut not gram negative bacteria.
- Replacement of both the proline residues with the pipecolic acid reduces the biological activity of the drug and the binding capacity of the drug with DNA.[3]
Methods of Synthesis
i. Dactinomycn is synthesized during the culture of Streptomyces antibioticus.
ii. It is purified with the help of chromatographic and crystallization methods.
TOTAL SYNTHESIS METHOD
i. Mixed anhydride frombenzyl-oxocarbonyl-L-N-methylvaline and isobutylchloroformate were treated with t-butyloxycarbonyl-L-threonine.
ii. Countercurrent distribution of the formed compound gave O-(benzyloxycarbonyl-L-N-methylvalyl)-N-t-butyloxycarbonyl-L-threonine.
iii. The catalytic hydrogenation of the above compound gave O- (L-N-methylvalyl)-N-t-butyloxycarbonyl-L-threonine.
iv. Condensation of the above compound with benzyloxycarbonylsarcosine with mixed carboxylic-carbonic anhydride method gave O- (benzyloxycarbonylsarcosy1-L-N-methylvaly1)-N-t-butyloxycarbonyl-L-threonine.
v. Condensation of the above formed compound with t-butyl D-valyl-L-prolinate by mixed anhydride method gave O-(benzyloxycarbonylsarcosyl-L- N – methylvalyl) – N – t- butyloxycarbonyl-L-threonyl-D-valyl-L-proline t-butyl ester.
vi. It was purified with counter current distribution method.
vii. The above formed compound was then treated with 0.4M solution of boron trifloride in glacial acetic acid to give chromatographically uniform yield.
viii. The crude product was purified using column chromatographic technique after treating the product with 2-nitro-3-benzyloxy-4-methylbenzoyl chloride.
ix. Product was then treated with di-p-nitrophenyl sulfite in pyridine to give nitrophenyl ester.
x. Benzoylcarbonyl group was removed by treatment with -5 N hydrogen bromide in dioxane.
xi. Purification of the product through column chromatography on Sephadex LH-20 in methanol will give cyclic pentapeptide lactone derivative.
xii. Catalytic hydrogenation followed by oxidation with potassium ferricyanite in methanol-phosphate buffer will give actinomycin D. [4]
Therapeutic Uses
Dactinomycin is used for the treatment of :
- Osteosarcoma
- Soft tissue sarcoma
- Locoregional solid tumors
- Gestational trophoblastic neoplasm
- Metastatic testicular tumors
- Ewing’s sarcoma
- Ovarian cancer
- Childhood rhabdomyosarcoma
- Wilms’ tumor
Side Effects
- Common side effects includes low blood counts, loss of hairs, vomiting, nausea, mouth sores, fatigue, liver problems, diarrhea and loss of fertility.
- Some people may suffer from side effects like loss of appetite, skin problems and sensitivity to light.
MCQs
Q.1 The term ‘DACMOZEN’ is associated with which drug?
a) Dactinomycin
b) Mitomycin
c) Bleomycin
d) Mitoxantron
Q.2 Predict the incorrect statement related to the therapeutic uses of drug Actinomycin D-
a) It is used for Wilms’ tumor
b) It is used for Hodgkin’s myloma
c) It is used for Osteosarcoma
d) It is used for Ewing’s sarcoma
Q.3 Match the following with respect to the SAR of drug Dactinomycin-
| i. Lactone ring | A. Biological activity of group |
| ii. Hydroxyproline group | B. Stabilizes the conformation of drug |
| iii. Peptide groups | C. Changes in the minimal inhibitory concentration of drug |
| iv. Pipecolic acid introduction | D. Decrease in the activity of drug |
a) i-C, ii-D, iii-B, iv-A
b) i-D, ii-B, iii-C, iv-A
c) i-B, ii-C, iii-A, iv-D
d) i-A, ii-B, iii-D, iv-C
Q.4 Which amongst the following drugs shows its effect through Binding with DNA and inhibition of RNA synthesis?
a) Cyclophosphamide
b) Methotrexate
c) Cytarabine
d) Dactinomycin
Q.5 Dactinomycin drug belongs to which class?
a) Progestins
b) Folate Antagonist
c) Antibiotics
d) Nitrogen mustards
Q.6 Which of the following is not a side effect of Actinomycin D?
a) Hair loss
b) Weight gain
c) Loss of appetite
d) Skin problems
Q.7 Phenoxazone ring structure is present in which drug?
a) Actinomycin D
b) Carmustine
c) Dacarbazine
d) None of the above
ANSWERS
1-a
2-b
3-c
4-d
5-c
6-b
7-a