AMOBARBITAL Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
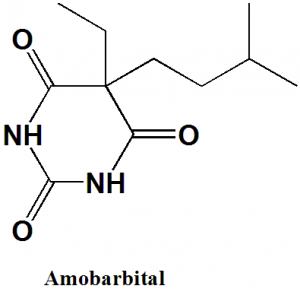
Amobarbital
IUPAC nomenclature
5-ethyl-5-(3-methylbutyl)-1,3-diazinane-2,4,6-trione.
Classification
Amobarbital is a barbiturate sedative-hypnotic.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 226.27 g/mol |
| 2 | Physical appearance | White crystalline solid |
| 3 | Melting point | 157°C |
| 4 | Octanol/water partition coefficient | 2.07 |
| 5 | Solubility | 1 g dissolves in: 1300 mL of water |
| 6 | Presence of ring | Pyrimidine |
| 7 | Number of chiral centers | 1 |
Mechanism of Action
- Amobarbital binds with GABAA receptor at either the α or ß subunit.
- Through binding, it potentiate the effect of GABA.
- It also decreases input resistance, depresses burst and tonic firing, especially in ventrobasal and intralaminar neurons, and also increases burst duration and mean conductance at individual chloride channel.
- Thus, both the amplitude and decay time of inhibitory post synaptic currents increases.
- It also blocks AMPA receptor, which is a subtype of glutamate receptor.
- Amobarbital also binds with neuronal nicotinic acetylcholine receptors.
Structure Activity Relationship
- Substitution of either of the 1,3-diazine nitrogens with aliphatic carbons retains the anticonvulsive properties.
- Esterification of the 5th-position substituents yields agents with analgesic activity but with weak hypnotic properties.
- Introduction of the polar functional group at the 5th– position yields compounds which are fully devoid of sedative-hypnotic or anticonvulsive activity.
- As the number of carbons at R2 carbon increases, the lipophillicity of the drug increases.
- Modification of the 2nd-position oxygen of the barbiturate backbone with sulfur atom yields thiobarbiturate derivatives with increased lipophillicity, shorter duration of action, faster time of onset compared to oxy-derivative. [1]
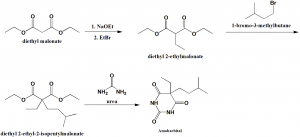
Method of synthesis
i. Diethyl malonate reacts with sodium ethoxide followed by reaction with ethylbromide to give diethyl 2-ethylmalonate.
ii. The compound undergoes reaction with 1-bromo-3-methylbutane to produce diethyl 2-ethyl-2-isopentylmalonate.
iii. The above formed compound on condensation with urea yields amobarbital.
Therapeutic Uses
Amobarbital is used for:
- Treatment of insomnia
- Anxiety
- Epilepsy
- Wada test
Side Effects
Side effects of Amobarbital are:
- Allergic reactions
- Depression
- Trouble breathing
- Tissue damage
- Feeling sleepy
- Somnolence
- Nervousness
- Hallucinations
- Nightmares
- Psychiatric disturbance
- Constipation
- Nausea
- Vomiting
- Bradycardia
- Hypotension
- Angiodema
- Hypersensitivity
- Liver damage
- Anemia
- Fever
MCQ
Q.1 What can be the correct IUPAC nomenclature of Amobarbital
a) 5-ethyl-5-(3-methylbutyl)-1,3-diazinane-2,4,6-trione
b) 3-ethyl-5-(5-methylbutyl)-1,3-diazinane-2,4,6-trione
c) 5-ethyl-5-(3-methylbutyl)-1,3-diazinane-2,4-dione
d) 3-ethyl-5-(5-methylbutyl)-1,3-diazinane-2,4-dione
Q.2 Which amongst the following statements is/are incorrect related to the SAR of Amobarbital?
I. Tri-keto form is least stable in aqueous solution.
II. 4,6-dialcoholic tautomeric forms are most stable in aqueous solution.
III. 5,5-disubstituted barbituric acid is the prime requirement for the barbituares to be sedative hypnotics.
IV. Esterification of either of the 1,3-diazine nitrogens decreases hypnotic activity.
a) III, IV
b) I, III, IV
c) I, III
d) I, II
Q.3 The correct order for the synthesis of drug amobarbital from diethyl malonate can be?
I. Reaction with ethyl bromide
II. Reaction with sodium ethoxide
III. Condensation with urea
IV. Reaction with 1-bromo-3-methylbutane
a) II – IV- III – I
b) II – I – IV – III
c) IV- II – I – III
d) III – II – I – IV
Q.4 Side effects of drug Amobarbital is/are?
a) Nervousness
b) Hallucinations
c) Bradycardia
d) All of the above
Q.5 Match the following drugs with their correct octanol/water partition coefficient-
| i. Amobarbital | A. 2.12 |
| ii.Nitrazepam | B. 2.25 |
| iii. Triazolam | C. 2.07 |
| iv. Clobazam | D. 2.42 |
a) i-A, ii-C, iii-B, iv-D
b) i-D, ii-C, iii-A, iv-B
c) i-A, ii-B, iii-C, iv-D
d) i-C, ii-B, iii-D, iv-A
Q.6 An example of drug from class barbiturate sedative-hypnotic?
a) Oxazepam
b) Amobarbital
c) Zolpidem
d) Chlordiazepoxide
Q.7 The type of ring system found in structure of amobarbital?
a) Diazepine
b) Benzene
c) Pyrimidine
d) Purine
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-a
2-d
3-b
4-d
5-d
6-b
7-c
REFERENCES
[1] Lemke TL, Zito SW, Roche VF, Williams DA. Essentials of Foye’s principles of medicinal chemistry. Wolters Kluwer; 2017, 473-474.