ATENOLOL Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
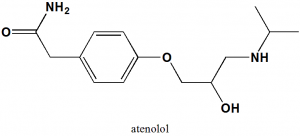
Atenolol
IUPAC nomenclature
(RS)-2-{4-[2-Hydroxy-3-(propan-2-ylamino)propoxy]phenyl}acetamide.
Classification
Atenolol is a cardioselective ß-blocker.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 266.34 g/mol |
| 2 | Physical appearance | Present in white crystalline powder form. |
| 3 | Melting point | 159°C |
| 4 | Solubility | Water solubility is 0.05M |
| 5 | Octanol/water partition coefficient | 0.16 |
| 6 | Presence of ring | Benzene |
| 7 | Number of chiral centers | 1 |
Mechanism of Action
i. Atenolol selectively binds with ß1-adrenergic receptors present in the vascular smooth muscles and the heart, and leads to block them.
ii. It leads to positive inotropic and chronotropic actions of endogenous catecholamines, which further results in inhibition of sympathetic stimulation.
iii. This activity decreases the heart rate, blood pressure and myocardial contractility.
iv. It also increases the AV node’s refractory period. [1]
Structure Activity Relationship
- Increasing the chain length of the side chain prevents appropriate binding of the required functional groups to the same receptors side.
- Side chain of aryloxypropanolamines can adopt a conformation that places the hydroxyl and amine groups into approximately the same position in space.
- Aryloxypropalonamines permits a close overlap with the arylethanomine side chain.
- Aryloxypropanolamines are more potent than aryloxyethanolamines. [2]
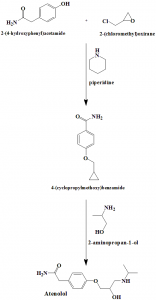
Method of synthesis
i. 2-(4-hydroxyphenyl)acetamide reacts with 2-(chloromethyl)oxirane in presence of piperidine to give 4-(cyclopropylmethoxy)benzamide.
ii. Latter compound reacts with 2-aminopropan-1-ol to give atenolol.
Therapeutic Uses
Atenolol is used for treatment of:
- Angina
- Hypertension
- Reduce the risk of death after a heart attack
Side Effects
Side effects of atenolol are:
- Chest pain worsening
- Uneven heartbeats
- Lightheadedness
- Shortness of breath
- Coldness of palm and feet
- Dizziness
- Tiredness
- Depression
MCQs
Q.1 Choose the correct option related to the correct physicochemical properties of drug Atenolol.
I. Molecular weight is 266.34 gm/mol.
II. It is present in yellow oily liquid form.
III. Melting point is 159°C
a) I, II, III
b) II, III
c) I, III
d) I, II
Q.2 Match the following of the drugs with their correct IUPAC names.
| i. Atenolol | A. (±)-[3-(9H-carbazol-4-yloxy)-2-hydroxypropyl][2-(2-methoxyphenoxy)ethyl]amine |
| ii. Labetolol | B. (RS)-2-Hydroxy-5-[1-hydroxy-2-[(4-phenylbutan-2-yl)amino]ethyl]benzamide |
| iii. Bisoprolol | C. (RS)-1-{4-[(2-Isopropoxyethoxy)methyl]phenoxy}-
3-(isopropylamino)propan-2-ol |
| iv. Carvedilol | D. (RS)-2-{4-[2-Hydroxy-3-(propan-2-ylamino)propoxy]phenyl}acetamide. |
a) i-B, ii-A, iii-C, iv-D
b) i-D, ii-A, iii-B, iv-C
c) i-A, ii-C, iii-D, iv-B
d) i-D, ii-A, iii-C, iv-B
Q.3 Correct steps for the mechanism of action of the drug Atenolol?
I. Positive inotropic and chronotropic actions of endogenous catecholamine
II. Blocking of ß1-adrenergic receptors
III. Decrease in heart rate, blood pressure and myocardial contractility.
a) I – III – II
b) II – I – III
c) III – I – II
d) II – III – I
Q.4 Correct sequence for True/false for the classification of the drug can be?
- Atenolol: selective ß1-adrenergic antagonist.
- Labetolol: mixed α/ß blocker
- Salmeterol: nonslective adrenergic agonist.
- Methyldopa: nonselective α-adrenergic antagonist
a) TTFF
b) TTFT
c) TFTF
d) FFTT
Q.5 Complete the following sentence related with structure activity relationship of atenolol-
‘aryoxypropanolamines are …………………. than aryloxyethanolamines.
a) more selective
b) less selective
c) more potent
d) less potent
Q.6 The correct sequence for the steps for synthesis of drug atenolol from 2-(4-hydroxyphenyl)acetamide?
I. Reaction with 2-(chloromethhyl)oxirane in presence of piperidine
II. Reaction with 2-aminopropan-1-ol
III. Reaction with 2-(chloromethhyl)oxirane in presence of ammonia.
a) I – II
b) III – II
c) II – I
d) II – III
Q.7 Side effect of drug atenolol?
a) Lightheadedness
b) Depression
c) Shortness of breath
d) All of the above
ANSWERS
1-c
2-d
3-b
4-a
5-c
6-a
7-d
REFERENCES
[1] Leigh PN, Jefferson D, Twomey A, Marsden CD. Beta-adrenoreceptor mechanisms in essential tremor; a double-blind placebo controlled trial of metoprolol, sotalol and atenolol. Journal of Neurology, Neurosurgery & Psychiatry. 1983 Aug 1;46(8):710-5. [2] Lemke TL, Williams DA, Foye WO. Principles of medicinal chemistry. Williams & Wilkins; 2017, 356. [3] Shrivastava A, Rathore P. Optimization and Validation of GC Method for Determination of Methanol as Organic Volatile Impurity in Atenolol Bulk Drug. World. 2013;1(4):54-8.