Atomic Absorption spectroscopy Principle, Instrumentation, Application & MCQ for GPAT, GATE, NET JRF
• Atomic Absorption Spectroscopy is a very common technique for detecting metals and metalloids in samples.
• It is very reliable and simple to use.
• It can analyze over 62 elements.
• It also measures the concentration of metals in the sample.
HISTORY:-
The first atomic absorption spectrometer was built by CSIRO scientist Alan Walsh in 1954. Shown in the picture Alan walsh(Ieft), with a spectrometer.
PRINCIPLE :-
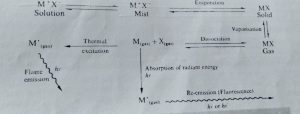
• The technique uses basically the principle that free atoms (gas) generated in an atomizer can absorb radiation at specific frequency.
• Atomic-absorption spectroscopy quantifies the
• absorption of ground state atoms in the gaseous states.
• The atoms absorb ultraviolet or visible light and make transitions to higher electronic energy levels. The analyte concentration is determined from the amount of absorption.
•Concentration measurements are usually determined from a working curve after calibrating the instrument with standards of known concentration.
•Atomic absorption is a very common technique for detecting metals and metalloids in environmental samples.
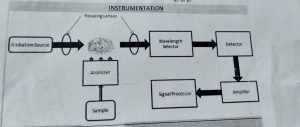
INSTRUMENTATION OF ATOMIC ABSORPTION SPECTROSCOPY :-
- Flame Atomizer
- Non flame Atomizer
- a. Electrothermal atomizer
- b. Cold vapour atomizer
- Flame Atomizers
- Flame used shall produced temp. in excess of 2000k.
- This requirement can only be meet by burning the fuel gas in oxidant gas which is usually air, nitrous oxide or oxygen or oxygen diluted with either N2 or argon.
-
FUEL OXIDANT TEMPERATURE Acetylene Air 2400-2700 Acetylene Oxygen 3300-3400 Acetylene Nitrous oxide 2900-3100 Hydrogen Air 2300-2400 - Liquid or dissolved sample :-
are typically used with flame atomizer. The sample solution is aspirated by a pneumatic nebulizer transformed into an aerosol which is introduced into a spray chamber where it is mixed with the flame gases and conditioned in a way that only the finest aersols droplets (< 10 μm) enter the flame.
- On top of the spray chamber is a burner head that produced a flame that is laterally long (usually 5-10 cm) and only a few mm deep. The radiation beam passes through this flame at its longest axis, and the flame gas flow rate may be adjusted to produced the highest concentration of free atoms.
- The burnner height may also be adjusted so that the radiation beam passes through the zone of highest atom cloud density in the flame resulting in the highest sensitivity.
Dis advantage of flame atomization technique :-
- Only 5-15% of the nebulised sample reaches the flame
- A minimum sample volume of 0.5-1.0 ml is need to give a reliable reading.
- Sample which are viscous require dilution with a solvent.
2.Non flame Atomizer :-
Hollow Cathode Lamp
•Cathode is in the form of a hollow cylinder made of the metal which has to be analysed
• Anode is made of tungsten filament
• They are sealed in a tube filled with inert gas like Neon or Argon
• A large voltage across anode and cathode causes the inert gas to ionize and form a plasma
• These ions are accelerated towards the cathode causing atoms to be sputtered off
• The ions and metal atoms are excited due to collisons
• They give off photons of a certain wavelength when they reach ground state
3.Nebuliser :-
The nebuliser forms a mist or aerosol of the sample
• This is done by forcing the sample at high velocities through a narrow tube
• The sample is mixed with a fuel and oxidant
• Commonly used fuel-oxidant mixtures are acetylene-air and acetylene-nitrous oxide.
4.Interferences :-
• Chemical interference:–
Presence of thermally stable compound that is not totally decomposed by the energy of the flame
High flame temperature provides energy for breakdown on interference .Addition of releasing agent which reacts with the
-
Ionization interferences :-
- Atoms of the samples are ionized causing reduction in number of electrons and absorbance.Addition of excess element like alkali elements, which gets ionized easily.
- Flame temperature may be reduced.
-
Matrix interference
- Due to viscosity, burning characteristics, surface tension of solvent Due to usage of different solvents in calibration and sample Addition of diluents to reduce viscosity
• Background absorptionLight scattering by particles in flame or absorption by undissociated molecules This must be measured and subtracted from final results Absorption of elements occurs as a narrow line whereas interference occurs over a broad rangeWorking :-
• The atoms of the solid are converted to gaseous state in the atomiser
• Radiation of specific wavelength is emitted by the hollow cathode lamp onto the gaseous atoms in the atomiser
• The monochromator focuses the specific wavelengths into the detector
• The detector finds the amount of light absorbed
• The concentration of atoms in the sample is directly proportional to the absorbanceAPPLICATION OF ABS :-
•Atomic absorption spectroscopy is one of the most widely used techniques for the determination of metals at trace levels in solution.
•Its popularity as compared with that of flame emission is due to its relative freedom from interferences by inter element effect and its relative insensitivity to various in flame temperature.
• Only bor the routine determination of alkali and alkaline earth metals is flame photometry usually preferred.
• Widely used for metal analysis in enviromental sample(air,water,and soil) and in biological fluid and tissues.
MCQ
1 .Choose the correct sequence of process during Atomization in atomic absorption spectroscopy? (GPAT 2019)
(a) Desolvation → Nebulization→ Dissociation →Volatilization → Ionization ion
(b) Nebulization → Desolvation → Volatilization →Dissociation → Ionization ion
(c) Desolvation → Nebulization → Volatilization → Dissociation →Ionization ion
(d) Nebulization →Volatilization → Desolvation →Dissociation →Ionization
2. In Atomic Absorption Spectroscopy, which of the following is the generally used radiation source?
(a) Tungsten lamp
(b) Xenon mercury arc lamp
(c) Hydrogen or deuterium discharge lamp
(d) Hollow cathode lamp
3. Which of the following is not a component of the emission system in Flame photometer?
(a) Burner
(b) Atomiser
(c) Fuel gases and their regulation
(d) Chopper
4. Which fuel is produced 3300-3400 temperature ?
(a) Acetylene+Air
(b)Acetylene+Oxygen
(c)Acetylene+Nitrous oxide
(d)Hydrogen+Air
5. Which fuel is produced 2400-2700 temperature ?
(a) Acetylene+Air
(b)Acetylene+Oxygen
(c)Acetylene+Nitrous oxide
(d)Hydrogen+Air
6. What is length and diameter of Hollow Graphic cylinder ?
(a) 50 mm and 9 mm
(b) 68 mm and 10 mm
(c) 100 mm and 80 mm
(d) 25 mm and 10 mm
7. Which instrument is used to convert sample in mist or aersol ?
(a) Atomizer
(b) Hollow cathode lamps
(c) Nebulizer
(d) Detector
ANSWER KEY
1.c
2.d
3.d
4.b
5.a
6.a
7.c
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in Online FREE GATE TEST: CLICK HERE
Participate in Online FREE CSIR JET JRF TEST: CLICK HERE
REFERENCE :-
TEXT BOOK PHARMACEUTICAL ANALYSIS THIRD EDITION BY DR.S.RAVI SANKAR (PG.NO. 27.1-27.8)