CHLORAZEPATE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Clorazepate
IUPAC nomenclature
7-Chloro-2,3-dihydro-2-oxo-5-phenyl-1H-1,4-benzodiazepine-3-carboxylic acid
Classification
Clorazepate is a benzodiazepine sedative-hypnotic.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 314.72 g/mol |
| 2 | Physical appearance | Fine white yellow crystalline powder |
| 3 | Melting point | 228-235°C |
| 4 | Octanol/water partition coefficient | 3 |
| 5 | Solubility | Very soluble |
| 6 | Presence of ring | Benzodiazepine, benzene |
| 7 | Number of chiral centers | 1 |
Mechanism of Action
i. Clorazepate converts into active metabolite desmethyl diazepam, which is partial agonist of GABA receptor.
ii. It binds with different receptors present in the brain and spinal cord.
iii. This increases the inhibitory effects of the GABA.
iv. Different subunits of GABA are responsible for anxiolytic, sedation, anticonvusant and anterograde amnesia effects.
Structure Activity Relationship
- Ring A should include an aromatic or heteroaromatic ring for binding with 5-phenyl-1,4-benzodiazepin-2-one derivatives.
- An electronegative group at 7-position of the ring A increases the functional anxiolytic activity.
- Substitutions at 6, 8 or 9 position with electronegative group on ring A will decrease the functional anxiolytic activity.
- When Heterocycles used as ring A, drug shows poor pharmacological activity.
- A proton-accepting group is essential on Ring B for binding with GABAA
- When the proton accepting group is present on the 2-position of the ring B, and is in coplanar spatial orientation with Ring A, maximum activity is observed.
- Replacement of oxygen with sulfur in ring B results in alteration in the selectivity for binding with GABA BZR subpopulations, but anxiolytic properties are maintained.
- There is no effect on agonist activity, but the antagonist activity dereases when methylene 3-position or imine nitrogen of the ring B is substituted.
- Derivatives having the 3-hydroxy moiety are fast excreted.
- Sterically large substituents on ring B, like tert-butyl group reduces the receptor affinity and the in vivo activity.
- 4,5-double bond and 4-position nitrogen is not essential for anxiolystic activity.
- BZR affinity is decreased if C=N bond is replaced with C-N bond.
- 5-phenyl ring C is not necessary for the binding with BZR.
- Substitution at the para position of the ring C decreases the agonist activity of the drug.
- There is no change observed in the agonist property of the drug when there is substitution at ortho position.
- When 1,2-bond f the ring C is annelated with an additional electron rich ring such as imidazole, affinity of the BZR increases. [1]
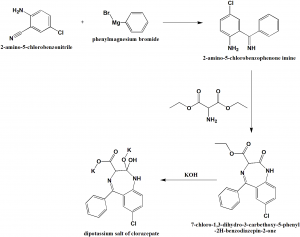
Method of synthesis
i. 2-amino-5-chlorobenzonitrile reacts with phenylmagnesium bromide to give 2-amino-5-chlorobenzophenone imine.
ii. On reaction with aminomalonic ester, a heterocyclization product, 7-chloro-1,3-dihydro-3-carbethoxy-5-phenyl-2H-benzodiazepin-2-one.
iii. On hydrolysis of the above formed compound with alcoholic solution of potassium hydroxide forms a dipotassium salt of chlorazepate.
Therapeutic Uses
Clorazepate is used for:
- Treatment of anxiety
- Treatment of Acute alcohol withdrawal
- Treatment of seizures
Side Effects
Side effects of Clorazepate are:
- Dizziness
- Drowsiness
- Blurred vision
- Constipation
- Upset stomach
- Dry mouth
- Fatigue
- Slurred speech
- Sleep disturbances
- Trouble urinating
- Tremors
- Change in libido
- Trouble in walking
- Clumsiness
- Depression
- Mood changes
- Suicidal thoughts
MCQ
Q.1 What can be the correct IUPAC nomenclature of Chlorazepate?
a) (S)-2-[1-[2-(2,3-dihydrobenzofuran-5-yl)ethyl] pyrrolidin-3-yl] -2,2-diphenyl-acetamide
b) (S)-2-[3-(Diisopropylamino)-1-phenylpropyl]-4-methylphenol.
c) 7-Chloro-2,3-dihydro-2-oxo-5-phenyl-1H-1,4-benzodiazepine-3-carboxylic acid
d) N,N-Dimethyl-2-[6-methyl-2-(4-methylphenyl)imidazo[1,2-a]pyridin-3-yl]acetamide hemitartrate
Q.2 Which amongst the following statements is/are incorrect related to the SAR of Chlorazepate?
I. Ring A should include an aromatic or heteroaromatic ring for binding with 5-phenyl-1,4-benzodiazepin-2-one derivatives.
II. An electronegative group at 7-position of the ring A increases the functional anxiolytic activity.
III. Substitutions at 6, 8 or 9 position with electronegative group on ring A will increase the functional anxiolytic activity.
IV. When Heterocycles used as ring A, drug shows poor pharmacological activity.
a) I, II
b) III
c) III, IV
d) II
Q.3 Corrects sequence of the steps involved in the synthesis of Chlorazepate from 2-amino-5-chlorobenzonitrile?
I. Hydrolysis
II. Reaction with aminomalonic ester
III. Reaction with phenylmagesium bromide
a) III- II – I
b) II – I – III
c) III – I – II
d) I – III – II
Q.4 Side effects of drug Clorazepate is/are?
a) Constipation
b) Drowsiness
c) Dry mouth
d) All of the above
Q.5 Match the following drugs with their correct molecular weights-
| i. Darifenacin | A.426.5 gm/mol |
| ii. Tolterodine | B.307.4gm/mol |
| iii. Chlorazepate | C. 325.5 gm/mol |
| iv. Zolpidem | D. 314.72 gm/mol |
a) i-C, ii-B, iii-A, iv-D
b) i-C, ii-B, iii-D, iv-A
c) i-A, ii-C, iii-D, iv-B
d) i-B, ii-A, iii-D, iv-C
Q.6 An example of drug from class Bezodiazepine sedative hypnotic?
a) Methysergide
b) Chlorazepate
c) Amphetamine
d) Tolterodine
Q.7 The type of ring system found in the structure of Chlorazepate?
a) Dihydrobenzofurane
b) Pyrrolopyrimidine
c) Benzodiazepine
d) Pyrrolopyrrole ring
Participate in Free Online Test for GPAT, Pharmacist,Drug Inspector
ANSWERS
1-c
2-b
3-a
4-d
5-c
6-b
7-c