CHLORDIAZEPOXIDE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
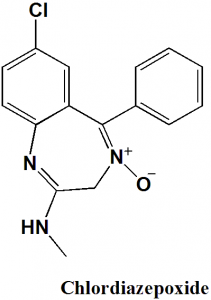
Chlordiazepoxide
IUPAC nomenclature
7-Chloro-2-methylamino-5-phenyl-3H-1,4-benzodiazepine-4-oxide
Classification
Chlordiazepoxide is a benzodiazepine sedative-hypnotic.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 299.75 g/mol |
| 2 | Physical appearance | Yellow crystalline powder; yellow plates from ethanol |
| 3 | Melting point | 236.2°C |
| 4 | Octanol/water partition coefficient | 2.44 |
| 5 | Solubility | Water solubility is 0.01 M |
| 6 | Presence of ring | Diazepine ring, phenyl ring |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
i. The drug binds to stereospecific benzodiazepine binding sites on GABA (A) receptor complexes within the CNS
ii. This increases the binding of the inhibitory neurotransmitter GABA to the GABA(A) receptor.
iii. There is enhancement in the GABA-mediated chloride influx through GABA receptor channels.
iv. Membrane hyperpolarization takes place.
v. There is net neuro-inhibitory effect which is having the properties such as sedative, hypnotic, anxiolytic and muscle relaxant. [1]
Structure Activity Relationship
- Ring A should include an aromatic or heteroaromatic ring for binding with 5-phenyl-1,4-benzodiazepin-2-one derivatives.
- An electronegative group at 7-position of the ring A increases the functional anxiolytic activity.
- Substitutions at 6, 8 or 9 position with electronegative group on ring A will decrease the functional anxiolytic activity.
- When Heterocycles used as ring A, drug shows poor pharmacological activity.
- A proton-accepting group is essential on Ring B for binding with GABAA
- When the proton accepting group is present on the 2-position of the ring B, and is in coplanar spatial orientation with Ring A, maximum activity is observed.
- Replacement of oxygen with sulfur in ring B results in alteration in the selectivity for binding with GABA BZR subpopulations, but anxiolytic properties are maintained.
- There is no effect on agonist activity, but the antagonist activity decreases when methylene 3-position or imine nitrogen of the ring B is substituted.
- Derivatives having the 3-hydroxy moiety are fast excreted.
- Sterically large substituents on ring B, like tert-butyl group reduces the receptor affinity and the in vivo activity.
- 4,5-double bond and 4-position nitrogen is not essential for anxiolystic activity.
- BZR affinity is decreased if C=N bond is replaced with C-N bond.
- 5-phenyl ring C is not necessary for the binding with BZR.
- Substitution at the para position of the ring C decreases the agonist activity of the drug.
- There is no change observed in the agonist property of the drug when there is substitution at ortho position.
- When 1,2-bond f the ring C is annelated with an additional electron rich ring such as imidazole, affinity of the BZR increases. [2]
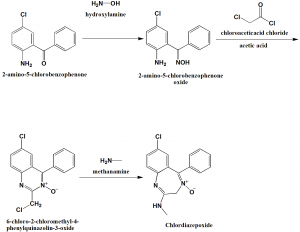
Method of synthesis
i. 2-amino-5-chlorobenzophenone is reacted with hydroxylamine to form 2-amino-5-chlorobenzophenone oxide.
ii. It cyclizes to 6-chloro-2-chloromethyl-4-phenylquinazolin-3-oxide on reaction with chloroacetic acid chloride in acetic acid.
iii. On reaction with primary amine such as methylamine, there is rearrangement, and the product formed is 7-chloro-2-methylamino-5-phenyl-3H-1,4-benzodiazepin-4-oxide or chlordiazepoxide. [3]
Therapeutic Uses
Chlordiazepoxide is used for:
- Treatment of anxiety
- Acute alcohol withdrawal
- Relieving fear
Side Effects
Side effects of Chlordiazepoxide are:
- Dizziness
- Nausea
- Constipation
- Blurred vision
- Headache
- Mood changes
- Slurred speech
- Clumsiness
- Trouble walking
- Change in libido
- Tremors
- Uncontrollable movements
- Muscle twitching
- Trouble urinating
- Sleep disturbances
- Fainting
- Abdominal pain
- Stomach pain
- Vomiting
- Fatigue
- Yellowing of eyes
- Dark urine
- Paleness of skin
- Sore throat
- Fever
- Allergic reactions
MCQ
Q.1 Binding of Chlordiazepoxide with acetylcholine muscarinic receptors results in?
a) Agonizing effect on muscarinic receptors
b) Antagonizing effect on muscarinic receptors
c) Do not produce any significant effect
d) Do not bind with muscarinic receptor
Q.2 Therapeutic use of drug Chlordiazepoxide is/are?
a) Treatment of Acute alcohol withdrawal
b) Relieving fear
c) Treatment of anxiety
d) All of the above
Q.3 Which amongst the following are the correct statements with respect to the SAR of drug Chlordiazepoxide?
I. Ring A should include an aromatic or heteroaromatic ring for binding with 5-phenyl-1,4-benzodiazepin-2-one derivatives.
II. An electronegative group at 7-position of the ring A increases the functional anxiolytic activity.
III. Substitutions at 6, 8 or 9 position with electronegative group on ring A will decrease the functional anxiolytic activity.
IV. When Heterocycles used as ring A, drug shows poor pharmacological activity.
a) I, IV
b) I, II, IV
c) I, II, III, IV
d) II, III, IV
Q.4 Type of ring structures present in the structure of Chlordiazepoxide?
a) Quiniclidine
b) Quinoline
c) Diazepine
d) All of the above
Q.5 Correct sequence for the True/False for the physiochemical properties of the drug Chlordiazepoxide?
I. Molecular weight is 299.75 gm/mol
II. Yellow crystalline compound
III. Melting point is 236.2°C
IV. Diazepine ring is present
a) TFTF
b) TTTT
c) FFFF
d)FFFT
Q.6 Correct statements for the IUPAC nomenclatures of the drugs are?
I. Solifenacin: (3R)-1-Azabicyclo[2 2 2]oct-3-yl (1S)-1-phenyl-3,4-dihydroisoquinoline-2(1H)-carboxylate
II. Zaleplon: N-(3-(3-cyanopyrazolo[1,5-a] pyrimidin-7-yl)phenyl)-N-ethylacetamide
III. Alprazolam: 7-Chloro-1,3-dihydro-1-methyl-5-phenyl-3H-1,4-benzodiazepin-2-one
IV. Chlordiazepoxide: 8-Chloro-1-methyl-6-phenyl-4H-[1,2,4]triazolo[4,3-a] [1,4]benzodiazepine
a) II, IV
b) I, II
c) I, III, IV
d) I, II, III, IV
Q.7 Match the following drugs with their correct classifications-
| i. Solifenacin | A. Barbiturate sedative-hypnotic |
| ii. Thiobarbital | B. Benzodiazepine sedative-hypnotic |
| iii. Chlordiazepoxide | C. Acetylcholine antagonist |
| iv. Zaleplon | D. Nonbenzodiazepine sedative-hypnotic |
a) i-C, ii-A, iii-B, iv-D
b) i-B, ii-C, iii-A, iv-D
c) i-D, ii-A, iii-C, iv-B
d) i-A, ii-C, iii-B, iv-D
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-d
2-d
3-c
4-c
5-b
6-b
7-a