Concept of dissolution: Dissolution mechanism and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam
Orally administered drugs can exert their pharmacologic actions only when they come into systemic circulation from their site of administration, and thus, drug absorption is an important prerequisite. Absorption of drug from solid oral dosage forms is mainly influenced by disintegration of the solid dosage form to yield granules, deaggregation of granules to yield fine particles, dissolution of drug from the fine particles into the solution and permeation of drug across the biomembrane to reach systemic circulation. Both dissolution and permeation are important parameters in the absorption of drug, with dissolution often being the rate-determining step for drugs with either low solubility (<1%) or that are given at high doses (e.g. griseofulvin and spironolactone).
Dissolution is the physicochemical process by which a solid substance enters the solvent phase to yield a solution and is a key prerequisite for any orally administered drug (as a solid dosage form) to be systemically effective.
Dissolution testing is the valuable tool to guide formulation development, assess product quality, monitor the manufacturing process and, in some cases, predict in vivo performance of solid oral dosage forms and as a surrogate measure for bioequivalence (BE) and provide biowaivers.
DISSOLUTION MECHANISM:
Drug dissolution is a multistep process involving heterogeneous reactions at the solid–liquid interphase. The mechanism of dissolution could be explained by two models:
(1)reactionlimited model and (2) diffusion-limited model.
1.Reaction-Limited Model: In a reaction-limited model, dissolution is considered to be a reaction between the undissolved species (solid) and the dissolution medium (liquid). In this model, the concentration of the undissolved species drives the dissolution rate and the solubility (Cs) is the result of chemical equilibrium. The reaction-limited dissolution can be explained by the interfacial barrier model and the Danckwert model.
Interfacial barrier model (Limited solvation theory) – This theory was proposed by Wilderman in 1909. According to this theory, at the interface of solid and liquid undergoing dissolution, an intermediate concentration can exist as a result of the solvation mechanism and is a function of solubility rather than diffusion. Thus, interfacial transport, rather than diffusion, is the limiting step in drug dissolution. The rate of dissolution
(D) controlled by the interfacial reaction is expressed as
dC/dt = K(Cs-Cb) (1)
where dC/dt is the rate of dissolution, K the effective interfacial transport constant, Cs the saturation solubility and Cb the bulk concentration at time t.
Danckwert model – This model for reaction-limited dissolution was proposed in 1951. According to this model, constantly renewed macroscopic packets of solvent, called eddy, reach the surface of the solid and absorb the molecule of the solute, delivering them to the bulk solution.
The concept of the Danckwert model for drug dissolution is expressed as
dC/dt = A(Cs-Cb) √γD/V (2)
where dC/dt is the rate of dissolution, A the surface area of the dissolving body, Cs the saturation solubility, Cb the bulk concentration at time t, J the rate of surface renewal, D the diffusion coefficient and V the volume of the dissolution medium.
- Diffusion-Limited Model or Film Theory: The diffusion layer model is a physical explanation for dissolution process, where the limiting step is the diffusion of molecules through a stagnant film of liquid (a hydrodynamic boundary layer) around the solid surface. According to this model, the dissolution rate depends on the hydrodynamic boundary layer, adhering closely to the surface of a solid particle that is to be dissolved. The diffusion-limited model has been exclusively based on experiments in a rotating or stationary disk apparatus or flow-through
cells under well-defined hydrodynamic conditions.
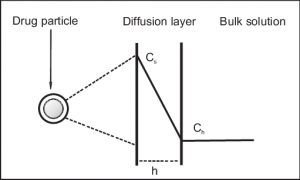
Fig 1 – Taken from Chapter 11 Drug Dissolution Gaurav K. Jain Theory and Practice of Physical Pharmacy
In the diffusion layer model, it is assumed that a stagnant film of liquid forms on the surface of the particle with a thickness h. The stagnant film constitutes a stationary diffusion layer in which the concentration of the drug is described by Cs. Beyond the thickness h is the bulk medium, where the concentration of the drug is Cb. The driving force for the dissolution, based on the diffusion theory, is the concentration gradient. The rate of diffusion is described by Fick’s law of diffusion and is expressed as
dC/dt = K∆C (3)
where dC/dt is the rate of diffusion, k the rate constant (s–1) and ‘C the concentration of solid in a solution at any point and at time t.
Noyes–Whitney Relationship – Noyes and Whitney described the quantitative analysis of the amount of drug dissolved from solid particles as a function of time. They found that the dissolution rate (dCb/dt) is a linear function of the difference between the bulk concentration (Cb) at time t and the saturation solubility (Cs). This statement can be formulated mathematically in the form of the Noyes–Whitney equation, as follows:
dCb/dt = Kd(Cs-Cb) (4)
where kd is the dissolution rate constant.
Furthermore, the Noyes and Whitney equation has been modified by Nernst and Brunner. According to them, kd is a constant being proportional to the diffusion coefficient, D, and the surface area of the dissolving body, S, and is inversely related to the volume of dissolution medium, V, and the thickness of the hydrodynamic boundary layer, h. The equation is written as
dC/dt = DS(Cs-Cb)/Vh (5)
Hixon–Crowell Cubic Root Law – In case of the Noyes–Whitney relationship, the surface area term in the equation remains constant throughout the dissolution process. However, for tablets, capsules, suspensions and powders, the size of the particles decreases as the drug dissolves and this decrease in size changes the effective area. Hixon and Crowell modified the Noyes–Whitney equation to take into account the changing surface area. The more appropriate equation (cubic root law) for powder dissolution is given by Hixon and Crowell, wherein the surface area is expressed in terms of weight (w).
W01/3-W1/3 = K2t (6)
where W0 is the initial weight, k2 a constant and t the time.
Multiple choice questions:
1. Absorption of drug from solid oral dosage forms is mainly influenced by _____ of the solid dosage form.
a)solubility
b)disintegration
c)particle size
d)partition coefficient
2.Which of the following are important parameters in the absorption of drug?
a)dissolution
b)permeation
c)both of these
d)none of these
3.Dissolution is the physicochemical process by which a solid substance enters the solvent phase to yield a solution and is a key prerequisite for any orally administered drug (as a solid dosage form) to be systemically effective.
a)true
b)false
4.Drug dissolution is a _____ process involving heterogeneous reactions at the solid–liquid interphase.
a)single step
b)multi step
c)linear
d)all of these
5.The mechanism of dissolution could be explained by
a)reactionlimited model
b)diffusion-limited model
c)both of these
d)none of these
6.Dissolution is considered to be a reaction between the undissolved species (solid) and the dissolution medium (liquid) in which of the following model?
a)Reaction-Limited Model
b)Diffusion-Limited Model or Film Theory
c)both of these
d)none of these
7.The reaction-limited dissolution can be explained by
a)Interfacial barrier model
b)Danckwert model
c)Noyes–Whitney Relationship
d)a and b
8.Interfacial barrier model is also known as
a)Limited solvation theory
b)Danckwert model
c)Noyes–Whitney Relationship
d)Hixon–Crowell Cubic Root Law
9.Which of the following is the limiting step in drug dissolution according to Interfacial barrier model?
a)interfacial transport
b)diffusion
c)solubility
d)particle size
10.Constantly renewed macroscopic packets of solvent, called eddy, reach the surface of the solid and absorb the molecule of the solute, delivering them to the bulk solution occurs according to which model?
a)Limited solvation theory
b)Danckwert model
c)Noyes–Whitney Relationship
d)Hixon–Crowell Cubic Root Law
11.The concept of the Danckwert model for drug dissolution is expressed as
a)dC/dt = K∆C
b)dC/dt = K(Cs-Cb)
c)W01/3-W1/3 = K2t
d)dC/dt = A(Cs-Cb) √γD/V
12.In Diffusion-Limited Model or Film Theory the limiting step is
a)interfacial transport
b)diffusion
c)solubility
d)particle size
13.The rate of diffusion is described by Fick’s law of diffusion and is expressed as
a)dC/dt = K∆C
b)dC/dt = K(Cs-Cb)
c)W01/3-W1/3 = K2t
d)dC/dt = A(Cs-Cb) √γD/V
14.Noyes–Whitney equation is expressed mathematically as
a)dC/dt = K∆C
b)dC/dt = K(Cs-Cb)
c)W01/3-W1/3 = K2t
d)dCb/dt = Kd(Cs-Cb)
15.Hixon and Crowell modified the Noyes–Whitney equation to take into account the changing surface area.
a)true
b)false
Solutions:
- b)disintegration
- c)both of these
- a)true
- b)multi step
- c)both of these
- a)Reaction-Limited Model
- d)a and b
- a)Limited solvation theory
- a)interfacial transport
- b)Danckwert model
- d)dC/dt = A(Cs-Cb) √γD/V
- b)diffusion
- a)dC/dt = K∆C
- d)dCb/dt = Kd(Cs-Cb)
- a)true
References:
- Gaurav K. Jain Theory and Practice of Physical Pharmacy, 1st edition Elsevier 2012, page no. 280-283.
- Martins Physical Pharmacy, 6th edition 2011, page no. 557-560.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test