Diazotization Titration: Principle, Reaction, Mechanism, Procedure and MCQ with Answer
Diazotization Titration :-
Diazotization Titration are used to determination of primary aromatic amine compound.
Principle :-
Reaction are performed in ice both at temp. 0-5 c.
End point is determined by the starch iodine paper or by potentiometric method.
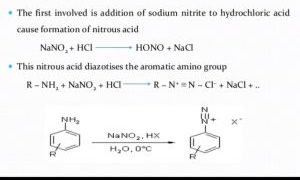
Mechanism :-
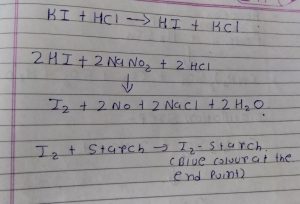
Procedure :-
preparation and standardization of 0.1 M NaNo2.
Take 7.5g of NaNo2 and dissolve 800ml of distilled water and finally make up the volume upto 1000 ml.
Standardized against sulphanilamide.
Take 0.3 g of sulphanilamide and dissolve in 2N HCL add 3g of kBr and titrate against prepare 0.1 M NaNo2 till the end point.
Application :-
It is used determination of alpha drug , sulphanilamide, chlorophenol, procaine etc.
MCQ
1. Which method are used to determination of primary amine ?
A. Diazotization Titration
B. Karl fischer titration
C. OFC
D. All of the above
2. Which temperature Is required to be performing Diazotization reaction ?
A. 25 to 30 c
B. O to 5 c
C. 10 to 15 c
D. All of the above
3. Which method is used to determination of end point of Diazotization Titration ?
A. Starch iodine paper
B. Potentiometric titration
C. A and B
D. None of this
4. Which method is used to determination of procaine ?
A. Diazotization Titration
B. Karl fischer titration
C. OFC
D. All of the above
5. How many grams of NaNO2 are dissolve in 800 ml distilled water ?
A. 6.5 g
B. 7.5 g
C . 6.0 g
D. 4.0 g
Answer key
1. A
2. B
3. C
4. A
5. B
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE