DOCETAXEL Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
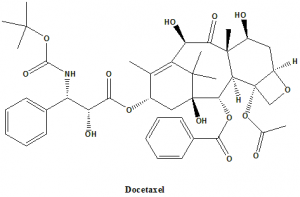
Docetaxel
IUPAC nomenclature
1,7β,10β-trihydroxy-9-oxo-5β,20-epoxytax-11-ene-2α,4,13α-triyl 4-acetate 2-benzoate 13-{(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hydroxy-3-phenylpropanoate}.
Classification
Docetaxel falls under the category of taxanes.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 807.9 g/mol |
| 2 | Appearance | Present in solid form |
| 3 | Melting point | 232°C |
| 4 | Solubility | Insoluble in water |
| 5 | Octanol water partition coefficient | 2.4 |
| 6 | Presence of ring | Taxane ring |
Mechanism of Action
i. Docetaxel got bound with beta-subunit of tubuline protein.
ii. This leads to the locking of the microtubules at their places and cell cannot use the microtubules in an effective way.
iii. This negatively affects the cell’s transportation mechanism and also inhibit the mitosis of the cell.
iv. Further, Docetaxel binds with stopping protein Bcl-2 and induces apoptosis in the cell. [1]
Structural Activity Relationship
- Phenyl moieties at C-3’N, C-3’ and C-2 positions are not necessary for the cytotoxicity and the anti-mitotic activity of the drug.
- Replacing the phenyl groups with t-butyl groups can increase the activity of the drug.
- Replacing the phenyl groups with t-butyl groups can increase the potency to induce apoptosis in the cell. It can also increase the bioavailability of the drug.
- Modification at the C-3 position of the C-2 benzoate with CN, N3, MeO, and Cl increases the anticancer activity against P-388 cell line.
- Introduction of a fluorine atom to the para position of 3′- phenyl decreased activity. [2]
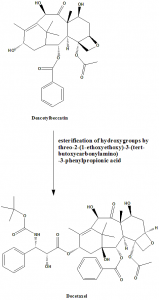
Methods of Synthesis
i. Protection of the 7- and 10-hydroxy groups of 10-deacetylbaccatin by 2,2,2,-trichloroethoxycarbonyl groups.
ii. Threo-2-(1-ethoxyethoxy)-3-(tert-butoxycarbonylamino)-3-phenylpropionic acid is prepared from tert-butyl cis-3-phenylglycidate (made by Darzens condensation of benzaldehyde with tert-butyl chloroacetate) by reaction with sodium azide, ester hydrolysis, and finally catalytic hydrogenation.
iii. 13-hydroxy group is esterified with threo-2-(1-ethoxyethoxy)-3-(tert-butoxycarbonylamino)-3-phenylpropionic acid. [3]
Therapeutic Uses
Docetaxel is used for the treatments of:
- Breast cancers
- Non-small cell lung cancer
- Advanced stomach cancers
- Head cancers
- Neck cancers
- Metastatic prostate cancers
- Small cell lung cancer
- Ovarian cancer
- Bladder cancer
- Pancreatic cancer
- Soft tissue sarcomas
- Melanomas
Side Effects
- Common side effects includes low WBS counts, low RBC counts, weight gains, swelling in the ankles and the abdominal regions, peripheral neuropathy, nausea, diarrhea, mouth sores, loss of hair, fatigue, weakness, infections and changes in the appearance of nails.
- Some people may suffer from side effects like vomiting, muscle and joint pains, low platelet counts, increase in the blood tests measuring liver functions and allergic reactions.
MCQs
Q.1 Taxotere and Docecad are the trade names of which drug?
a) Paclitaxel
b) 5-FU
c) Docetaxel
d) Vinblastine
Q.2 Which amongst the following statements is/are incorrect related to the SAR of Docetaxel
I. Introduction of a fluorine atom to the para position of 3′- phenyl decreased activity.
II. Modification at the C-3 position of the C-2 benzoate with CN, N3, MeO, and Cl increases the anticancer activity.
III. Phenyl moieties at C-3’N, C-3’ and C-2 positions are not necessary for the cytotoxicity and the anti-mitotic activity of the drug.
a) None of the above
b) All of the above
c) Only I & II
d) Only I & III
Q.3 The correct order for the mechanism of action of Docetaxel can be?
I. locking of the microtubules at their places
II. Docetaxel got bound with beta-subunit of tubuline protein
III. Cell’s transportation mechanism is affected
a) I – III – II
b) I – II – III
c)III – I – II
d)II – I – III
Q.4 The drug Docetaxel is mainly used for?
a) Bradycardia
b) GIT infections
c) Given as a sedative
d) Breast cancer
Q.5 Match the drugs with the correct classification.
| i. Docetaxel | A. Ethylinimine cytotoxic drug |
| ii. Dacarbazine | B. Folate antagonist |
| iii. Thiotepa | C. Taxanes |
| iv. Methotrexate | D. Triazine |
a) i-C, ii-D, iii-A, iv-B
b) i-B, ii-D, iii-A, iv-C
c) i-C, ii-A, iii-D, iv-B
d) i-D, ii-C, iii-B, iv-A
Q.6 How many statements below are true with respect to the side effects of the drug Docetaxel?
- Increase in blood tests measuring liver functions.
- Higher RBC counts
- Higher WBC counts
- Higher platelets count
a) 0
b) 1
c) 2
d) 3
Q.7 The type of ring system found in Docetaxel is?
a) No ring is present
b) Taxane
c) Pteridine ring system
d) Oxazophospherine ring system
ANSWERS
1-c
2-a
3-d
4-d
5-a
6-c
7-b
@copyright with Pharmacophore Edulabs Solution