Dosage form necessities and additives: Antioxidants, Properties of Antioxidants for Pharmaceutical Use and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam
Antioxidants: Such materials, when added to a product, prevent the active from degrading in the presence of oxygen or peroxides.
Examples – Butylated Hydroxyanisole (BHA), Butylated Hydroxytoluene (BHT), Citric acid, Sodium Metabisulfite
1.Butylated hydroxyanisole:
INN: BHA; Butylhydroxyanisolum; Butylated hydroxyanisole
Synonyms: BHA; Sustane; Tenox
Chemical Name: 2-tert-Butyl-4-methoxyphenol
Molecular Formula: C11H16O2
Structure:
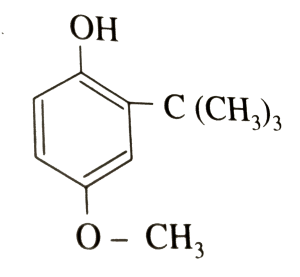
Description: White or slightly yellow, waxy solid, having a faint, characteristic odor
Properties: Insoluble in water; 1 g in 4 mL alcohol, 2 mL chloroform or 1.2 mL ether; antimicrobial activity against molds and gram positive bacteria.
Storage: In a tightly sealed container, which will protect the material from light. Exposure to light causes discoloration and decreased activity. and decreased activity.
Incompatibilities: Avoid use with peroxides and permanganates; contact with oxidizing agents may result in spontaneous combustion. Avoid iron salts, as these reduce effectiveness.
Uses: As an antioxidant and antimicrobial agent in liquid and semi-solid preparations.
2.Butylated hydroxytoluene:
INN: Butylhydroxytoluenum; Butylated hydroxytoluene
Synonyms: Agidol, BHT
Chemical Name : 2,6-Di-tert-butyl-4-methylphenol
Molecular Formula: C15H24O
Structure:
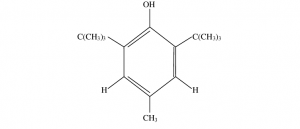
Description: White, tasteless crystals with a mild odor; stable in light or air.
Properties: Melts at 70°C; insoluble in water; 1 g in 4 mL alcohol, 1.1 mL chloroform, or 1.1 mL ether.
Incompatibilities: Avoid use with peroxides and permanganates, contact with oxidizing agents may result in spontaneous combustion. Avoid iron salts, as these reduce effectiveness.
Uses: As an antioxidant.
3.Citric Acid:
INN: Citric acid
Synonyms: 2-Hydroxypropane-1,2,3-tricarboxylic acid monohydrate
Chemical Name : 1,2,3-Propanetricarboxylic acid, 2-hydroxy-,Citric acid C6H8O7; monohydrate C6H8O7 .H2O
Molecular Formula: Citric acid C 6H8O7; monohydrate C 6H8O7 .H2O
Structure:

Description: A white, granular to fine crystalline powder; odorless; strongly acid taste.
Properties: 1 g in 0.5 mL water, 2 mL alcohol; density 1.5 g/cc.
Uses: As an acidifier, antioxidant, buffering agent, and sialogogue.
4.Sodium Metabisulfite:
INN: Sodium metabisulfite; Natrii metabisulfis
Synonyms: Disodium disulfite; disodium pyrosulfite; sodium pyrosulfite
Chemical Name :Sodium pyrosulfite
Molecular Formula: Na2S2O5
Description: White crystals or white to yellowish crystalline powder with an odor of sulfur dioxide.
Properties: Solubility 1 g in 2 mL water; slightly soluble in alcohol; freely soluble in glycerin; 5% solution pH 3.5–5.0; melting point 150°C.
Incompatibilities: Phenylmercuric acetate, possible rubber caps of multi-dose vials.
Uses: As an antioxidant.
Multiple choice questions:
1.Such materials, when added to a product, prevent the active from degrading in the presence of oxygen or peroxides are called as
a)antioxidants
b)preservatives
c)chelating agents
d)surface active agents
2.Which of the following are examples of antioxidants?
a)Butylated Hydroxyanisole
b)Butylated Hydroxytoluene
c)Citric acid
d)All of these
3.BHA stands for
a)Butylated Hydroxyanisole
b)Butyl Hydroxyanisole
c)Butylated Hydroxyanithole
d)Butylated Hydroxide anisole
4.BHT stands for
a)Butyl Hydroxytoluene
b)Butylated Hydroxytoluene
c)Butylated Hydrotoluene
d)Butylated Hydroxide toluene
5.What are the synonyms of Butylated hydroxyanisole?
a)BHA
b)Sustane
c)Tenox
d)All of these
6.2-tert-Butyl-4-methoxyphenol is the chemical name of
a)Butylated Hydroxyanisole
b)Butylated Hydroxytoluene
c)Citric acid
d)Sodium Metabisulfite
7.Which of the following are physical properties of BHA?
a) waxy solid
b)stable in light or air
c)strongly acid taste
d)odor of sulfur dioxide
8.2,6-Di-tert-butyl-4-methylphenol is the chemical name of
a)Butylated Hydroxyanisole
b)Butylated Hydroxytoluene
c)Citric acid
d)Sodium Metabisulfite
9.BHT melts at
a)40 degrees
b)50 degrees
c)60 degrees
d)70 degrees
10.Incompatibilities of BHT are
a)with peroxides and permanganates
b)contact with oxidizing agents
c)with iron salts
d)all of these
11.Citric Acid is used as
a)an acidifier
b)antioxidant
c)buffering agent
d)all of these
12.Which of the following is/are properties of citric acid?
a) waxy solid
b)stable in light or air
c)strongly acid taste
d)odor of sulfur dioxide
13.What are the synonyms of Sodium Metabisulfite?
a)Disodium disulfite
b)disodium pyrosulfite
c)sodium pyrosulfite
d)all of these
14.Na2S2O5 is the molecular formula of
a)Butylated Hydroxyanisole
b)Butylated Hydroxytoluene
c)Citric acid
d)Sodium Metabisulfite
15.Incompatibilities of Sodium Metabisulfite is/are with
a)Phenylmercuric acetate
b)Possible rubber caps of multi-dose vials
c)Both of these
d)None of these
Solutions:
- a)antioxidants
- d)All of these
- a)Butylated Hydroxyanisole
- b)Butylated Hydroxytoluene
- d)All of these
- a)Butylated Hydroxyanisole
- a) waxy solid
- b)Butylated Hydroxytoluene
- d)70 degrees
- d)all of these
- d)all of these
- c)strongly acid taste
- d)all of these
- d)Sodium Metabisulfite
- c)Both of these
References:
- Remington Essential of Pharmaceutics, 1st edition 2013, page no. 683,688,690, 700.
- Raymond C Rowe handbook of Pharmaceutical excipients, 6th edition 2009, page no. 73, 75, 181-183, 654-656.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test