DOXORUBICIN Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Doxorubicin
IUPAC nomenclature
(7S,9S)-7-[(2R,4S,5S,6S)-4-Amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione.
Classification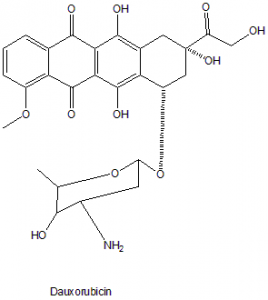
Doxorubicin falls under the category of Anthracyclic antibiotic cytotoxic drug. [1]
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 543.5 g/mol |
| 2 | Appearance | Red crystalline solid |
| 3 | Melting point | Range between 229-231°C |
| 4 | Solubility | Soluble in water |
| 5 | Octanol water partition coefficient | 1.27 |
| 6 | Presence of ring | Anthraquinone ring system. |
Mechanism of Action
i. Doxorubicin firms complexes with DNA by intercalation between base pairs.
ii. The drug then stabilizes DNA-topoisomerase II and thus topoisomerase activity is inhibited.
iii. Relegation of the DNA is prevented.
iv. Cell death. [2]
Structural Activity Relationship
- Substitution at 2nd position decreases the biological activity of drug.
- Presence of any substituent at R2 position also decreases the biological activity of drug.
- Biological activity can be increased by substitution at 3rd
- 8th position has direct relationship with the biological activity of drug and thus, substitution at 8th position can increase the biological activity of drug.
- Substitution at 1st and 7th position will have negative impact on the biological activity of the drug.[3]
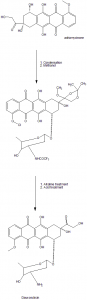
Methods of Synthesis
i. In the first step, adriamycinone converts into dioxolone derivative.
ii. It is then condensed with N-O-ditriflouroacetyl-alpha-daunosaminyl chloride.
iii. The formed compound is then treated with methanol to remove the O-triflouroacetyl group.
iv. First alkaline and then an acid treatment is given to the compound to give doxorubicin.
Therapeutic Uses
Doxorubicin is used for the treatment of :
- Waldenstorm macroglobulinemia
- Wilm’s tumor
- Uterine sarcoma
- Transitional cell bladder cancer
- Thyroid cancer
- Thyomas
- Soft tissue sarcoma
- Small cell lung cancer
- Ovarian cancer
- Neuroblastoma
- Multiple myeloma
- Kidney cancer
- Liver cancer
- Non-Hodgkin’s lymphoma
- Hodgkin’s lymphoma
- Head and neck cancer
- Gastric cancer
- Endometrial cancer
- Breast cancer
- Bone sarcoma
- Acute myeloblastic leukemia
- Acute lymphoblastic leukemia
Side Effects
- Common side effects includes low blood counts, pain, nausea, vomiting and hair loss.
- Some people may suffer from side effects like eyes watering, mouth sores, nail darkening, skin darkening and infertility.
MCQs
Q.1 What can be the correct Trade name for Doxorubicin?
a) Irinotel
b) Dacmozen
c) Oncotron
d) Adriamycin
Q.2 Which amongst the following statements is/are incorrect related to the SAR of Doxorubicin?
I. Substitution at 2nd position will increase the biological activity of drug.
II. Substitution at 1st and 7th position will have negative impact on the biological activity of the drug.
III. Substitution at 1st and 7th position will have negative impact on the biological activity of the drug.
a) I, II & III
b) I & II
c) I
d) II & III
Q.3 The correct order for the mechanism of action of Doxorubicin can be?
I. Stabilization of DNA-Topoisomerase II complex
II. Intercalation between DNA base pairs
III. Relegation of DNA is prevented
a) I – II – III
b) II – I – III
c) III – I – II
d) III – II – I
Q.4 The drug Doxorubicin is mainly used for?
a) Treatment of cancers
b) As an antiviral drug
c) Antiarrytthmic drug
d) Both a) and c)
Q.5 Match the drugs with the correct classification.
| i. Doxorubicin | A. Aromatase inhibitors |
| ii. Busulfan | B. Vinca alkaloids |
| iii. Vinblastin | C. Alkyl sulfonate |
| iv. Exemestane | D. Antibiotics |
a) i-A, ii-B, iii-C, iv-D
b) i-D, ii-C, iii-B, iv-A
c) i-C, ii-D, iii-B, iv-A
d) i-D, ii-C, iii-A, iv-B
Q.6 How many statements below are true with respect to the side effects of the drug Doxorubicin?
- Eye watering
- Loss of hairs
- Low blood counts
- Nausea and vomiting
a) 2
b) 3
c) 4
d) 1
Q.7 The type of ring system found in Doxorubicin is?
a) No ring system present
b) Pyrimidine ring system
c) Purine ring system
d) Anthraquinone ring system
ANSWERS
1-d
2-c
3-b
4-a
5-b
6-c
7-d