ETHINYLESTRADIOL Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
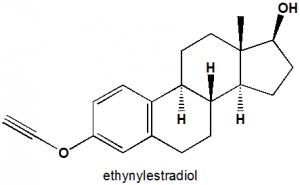
Ethinylestradiol
IUPAC nomenclature
(8R,9S,13S,14S,17R)-17-ethynyl-13-methyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthrene-3,17-diol.
Classification
Ethinylestradiol is classified as an estrogen drug, altering the hormonal milieu. [1]
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 296.4 g/mol |
| 2 | Appearance | White to creamy white powder |
| 3 | Melting point | 183°C |
| 4 | Solubility | 11.3 mg per liter in water |
| 5 | Octanol/water partition coefficient | 3.67 |
| 6 | Presence of ring | Naphthalene and indinol ring |
| 7 | Number of chiral centers | 5 |
Mechanism of Action
i. The drug will diffuse into the target cells and interact with protein receptors.
ii. Target cells for the drug includes pituitary, hypothalamus, mammary gland and female reproductive tract.
iii. This increases the synthesis of sex hormone binding globulin, thyroid binding globulin and other serum proteins. Further, the FSH is suppressed. [2]
Structural Activity Relationship
- Oxygen at C11 is important for the activity of drug.
- Alcoholic oxygen at C11 is superior than the ketonic one.
- Introduction of 17-alpha-hydroxy group increases the activity.
- Introduction of 6-alpha-F or 9-alpha-F group can also increase the activity of drug.
- Double bond at C1 position increases anti-inflammatory activity of the drug.
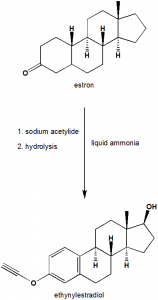
Method of synthesis
i. Estrone is reacted with sodium acetylide in liquid ammonia.
ii. Above formed complex then undergoes hydrolysis to give ethinylestradiol.
Therapeutic Uses
- Used as a contraceptive
- Used as menopausal hormone therapy
- Used as feminizing hormone therapy for transgender women
- For the treatment of hypogonadism is women
- For prevention of osteoporosis in women
- For the treatment of prostate cancer in men
- For the treatment of breast cancer in women
Side Effects
- Common side effects of Ethinylestradiol include breast tenderness, headache, weight gain, dizziness, nausea, fluid retention, etc.
- Less common side effects are sexual dysfunction, infertility, hypogonadism, feminization and gynacomastia.
MCQs
Q.1 Number of chiral centers in the structure of ethinylestradiol?
a) 1
b) 3
c) 5
d) 7
Q.2 Which amongst the following statements is/are incorrect related to the SAR of ethnylestradiol?
I. Oxygen at C11 is not important for the activity.
II. Introduction of 17-alpha-hydroxy group increases the activity
III. Introduction of 17-alpha-hydroxy group decreases the activity
a) I & III
b) I & II
c) I only
d) All statements are correct
Q.3 The correct order for the mechanism of action of ethinylestradiol can be?
I. Target cells for the drug includes pituitary, hypothalamus, mammary gland and female reproductive tract.
II. Increase in the synthesis of sex hormone binding lobulin, thyroid binding globulin and other serum proteins. Further, the FSH is suppressed.
III. The drug will diffuse into the target cells and interact with protein receptors.
a) I – III – II
b) II – III – I
c) I – II – III
d) III – I – II
Q.4 The drug ethinylestradiol is used for?
a) The treatment of breast cancer
b) Menopausal hormone therapy
c) Used as a contraceptive
d) All of the above
Q.5 Match the drugs with the correct classification.
| i. Prednisolone | A. Selective estrogen receptor down regulators |
| ii. Ethinylestradiol | B. Estrogens |
| iii. Tamoxifen | C. Glucocorticoids |
| iv. Fulvestrant | D. Selective estrogen receptor modulator |
a) i-B, ii-D, iii-C, iv-A
b) i-B, ii-A, iii-C, iv-D
c) i-D, ii-A, iii-C, iv-B
d) i-C, ii-B, iii-A, iv-D
Q.6 How many statements below are true with respect to the side effects of the drug Ethinylestradiol?
- Breast tenderness is a common side effect.
- Weight loss may occur in the patient taking the drug.
- Infertility is a less common side effect.
- Feminization is a side effect of the drug
a) 1
b) 2
c) 3
d) 4
Q.7 The type of ring system found in Ethinylestradiol?
a) Naphthalene
b) Indole
c) Pteridine
d) None of the above
ANSWERS
1-c
2-a
3-b
4-d
5-d
6-c
7-a