Exchange of oxygen and carbon dioxide in External and Internal Respiration; MCQ With Answer
The exchange of oxygen and carbon dioxide between alveolar ducts and pulmonary capillaries occurs through passive diffusion which in relation with the two laws, the Dalton’s law and Henry’s law.
According to the Dalton’s law the partial pressure determines the movement of oxygen and carbon dioxide between the atmosphere and lungs , lungs and blood in pulmonary capillaries , pulmonary capillaries and tissue cells. Diffusion of these gases is from the area where the partial pressure is higher to the area where it is lower; and greater the difference in partial pressure results in more faster is rate of diffusion .
While the Henry’s law states that the ability of gases to stay in liquid is directly proportional to the partial pressure i.e greater the partial pressure greater is the stability and solubility in water. For eg the stability of oxygen in blood plasma is very less because the poor solubility of oxygen in water also due to its low partial pressure in comparison with carbon dioxide.
External and Internal Respiration:-
External respiration refers to diffusion of oxygen fron alveoli to the blood in pulmonary capillaries and movement of CO2 in opposite direction. While the internal respiration is movement of oxygen from blood in systemic capillaries to the cells and tissues.
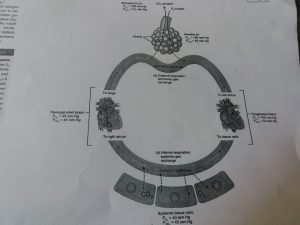
As seen in the above mentioned diagram , the oxygen is inhaled , reaches the alveoli and from alveoli it reaches the left atrium because the partial pressure of oxygen in alveoli is 105 mm Hg and in left atrium it is 100 mm Hg and in respect to the Dalton’s law gases diffuse from area of high partial pressure to the lower area, again during internal respiration the oxygen moves from blood in systemic capillaries to the cells and tissues where in the partial pressure is 100 mm Hg in left atrium and 40 mmHg in cells. In the same way movement of carbon dioxide also takes place but in opposite direction.
Factors affecting the rate of exchange of gases:-
1. Partial pressure difference of gases:- greater the difference in partial pressure, more rapid is the diffusion
2. Surface area:- greater the surface area increase the rate of diffusion
3. diffusion distance:- Smaller the diffusion distance, more rapid is the diffusion
4. molecular weight and solubility of gases:- Lower the molecular weight, faster is the exchange while on the other hand greater the solubility, more is the diffusion.
MULTIPLE CHOICE QUESTIONS(MCQs)
1. Which process facilitates the exchange of oxygen and carbon dioxide?
A. simple diffusion B. osmosis
C. active diffusion D. passive diffusion
2. Which law states that “each gas in a mixture of gases exerts specific pressure”
A. Henry’s law B. Boyle’s law
C. Dalton’s law D. Bohr’s law
3. Even though the air contains 79% nitrogen, Why this gas has no effect on body fluids?
A. high partial pressure B. Low solubility in blood
C. HIGH SOLUBILITY D. low partial pressure
4. Which of the following factors affect the gas exchange
A. MOLECULAR WEIGHT B. diffusion distance
C. Surface area D. all of the above
5. How much is the partial pressure of CO2 in cells and tissues?
A. 40 mm Hg B. 100 mmHg
C. 45mm Hg d. 105 mm Hg
6. Which of the following statement is NOT true?
A. Diffusion distance is directly proportional to rate of exchange
B. External respiration is carried out by pulmonary capillaries
C. Internal respiration is carried out by systemic capillaries
D. Molecular weight is inversely proportional to rate of exchange
7. Which of the following is inversely proportional to the rate of exchange?
A. Partial pressure difference B. Solubility
C. Surface area for gas exchange D. none of the above
8. Match the following
1. amount of gas dissolve is proportional (a) increases diffusion
To the partial pressure
2. partial pressure proportional to the (b) Henry’s law
Rate of diffusion
3. greater diffusion distance (c) Dalton’s law
4. greater surface area (d) decreases diffusion
9. The condition in which excess amount of nitrogen is dissolved in plasma is known as?
A. Nitrogen narcosis B. decompression sickness
C. Insomnia D. Hypercapnia
10. Which of the following is not the symptom of high altitude sickness
A. Fatigue B. Dizziness
C. Hypoxia D. Nausea
ANSWERS:-
1. simple diffusion
2. Dalton’s law
3. low solubility in blood
4.all of the above
5. 40 mm Hg
6. diffusion distance is directly proportional to rate of exchange
7. none of the above
8. 1 – b 2 – c 3 – d 4 – a
9. nitrogen narcosis
10. hypoxia
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
REFRENCES:- 1. Ross and Wilson-Anatomy and physiology in health and illness; 12th edition; page no.-:
2. Gerard J. Tortora -Principles of anatomy and physiology; edition twelfth ; page no.-: 896-899.