FAVIPIRAVIR Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
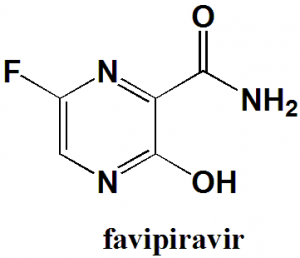
Favipiravir
IUPAC nomenclature
6-fluoro-3-hydroxypyrazine-2-carboxamide.
Classification
Favipiravir is an antiviral medication. It is a modified pyrazine analogue.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 157.1 g/mol |
| 2 | Physical appearance | Light yellow to yellow solid |
| 3 | Melting point | 187-193°C |
| 4 | Solubility | Slightly soluble in water |
| 6 | Presence of ring | Pyrazine |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
Favipiravir interacts with RNA dependent RNA polymerase. It prevents the elongation of the RNA strand and viral proliferation by incorporating into nascent RNA strand. Thus, it selectively inhibits RNA polymerase and prevents replication of the viral genome.
Structure Activity Relationship
- Modified 2’C-methyl-NTP analogs causes immediate chain termination.
- Smaller compounds with pyrazine ring are docked deeper but relatively smaller pocket of VP35.
- For proper binding with VP35 IID, essential benzene rings and pyrrilidinone scaffold are required. [1]
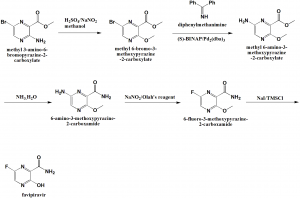
Method of synthesis
i. Methyl 3-amino-6-bromopyrazine-2-carboxylate undergoes reaction with sodium nitrite in presence of acid to produce methyl 6-bromo-3-methoxypyrazine-2-carboxylate.
ii. The above compound undergoes reaction with diphenylmethanamine to methyl 6-amino-3-methoxypyrazine-2-carboxylate.
iii. It then undergoes reaction with ammonia water to produce 6-amino-3-methoxypyrazine-2-carboxamide.
iv. Compound is thereafter reacted with sodium nitrite in presence of Olah’s reagent to produce 6-fluoro-3-methoxypyrazine-2-carboxamide.
v. The above formed compound is then reacted with sodium iodide in TMSCl to yield favipiravir.
Therapeutic Uses
Favipiravir is used for the treatment of Influenza
Side Effects
Side effects of Favipiravir include the harm to the baby when the drug is given to the pregnant mother.
MCQ
Q.1 What can be the correct IUPAC nomenclature of the drug Favipiravir?
a) 6-fluoro-3-hydroxypyrazine-2-carboxamide
b) 22,23-dihydroavermectin
c) 6-fluoro-3-hydroxypyrazine-2-sulfonic acid
d) None of the above
Q.2 Which amongst the following statements is/are incorrect related to the SAR of ivermectin?
I. Modified 2’C-methyl-NTP analogs causes immediate chain termination.
II. Smaller compounds with pyrazine ring are docked deeper but relatively smaller pocket of VP35.
III. For proper binding with VP35 IID, essential benzene rings and pyrrilidinone scaffold are required.
a) I
b) II
c) III
d) None
Q.3 Type of ring present in the structure of favipiravir ?
a) Furane
b) Pyrazine
c) Purine
d) No ring present
Q.4 Side effects of drug favipiravir is/are?
a) Harm to fetus of pregnant lady
b) Hair loss
c) Hallucinations
d) All of the above
Q.5 Match the following drugs with their correct molecular weights-
| i. Favipiravir | A. 335.9 gm/mol |
| ii. Diazepam | B. 284.7 gm/mol |
| iii. Amobarbital | C. 157.1 gm/mol |
| iv. Hydroxychloroquine | D. 226.7 gm/mol |
a) i-C, ii-B, iii-D, iv-A
b) i-A, ii-B, iii-C, iv-D
c) i-B, ii-D, iii-C, iv-A
d) i-D, ii-A, iii-C, iv-B
Q.6 An example of drug from class antiviral pyrazine analogue ?
a) Fluorouracil
b) Mitomycin
c) Fludarabine
d) Favipiravir
Q.7 Number of chiral centers present in the structure of favipiravir is?
a) 0
b) 2
c) 5
d) 6
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-a
2-d
3-b
4-a
5-a
6-d
7-a