IPRATROPIUM BROMIDE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Ipratropium bromide
IUPAC nomenclature
[8-methyl-8-(1-methylethyl)- 8-azoniabicyclo[3.2.1] oct-3-yl] 3-hydroxy-2-phenyl-propanoate.Classification
Ipratropium is an acetylcholine antagonist. It is a muscarinic antagonist.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 412.4 g/mol |
| 2 | Physical appearance | Almost white crystalline powder |
| 3 | Melting point | 231°C. |
| 4 | Solubility | Freely soluble in water |
| 5 | Presence of ring | Present |
| 6 | Number of chiral centers | 5 |
Mechanism of Action
i. The drug inhibits the parasympathetic nervous system in the airways.
ii. Ipratropium stops the activity of acetylcholine in the smooh muscles preventing the contraction and relaxed airways.
iii. This leads to bronchodilation and a fewer secretions. [1]
Structure Activity Relationship
- Either R1 or R2 must be heterocyclic or carbocyclic.
- The R3 group can be hydrogen, hydroxyl, hydroxymethyl or amide.
- Most potent derivatives has X as an ester.
- X can also be either oxygen or absent completely.
- The N substituent can be quaternary ammonium salt or tertiary amine or both with different alkyl groups.
- Maximum potency obtained when the distance between the ring substituted carbons is 2 carbon units.
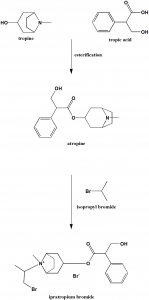
Method of synthesis
i. Tropic acid and tropine reacts to give atropine.
ii. Treating atropine with isopropyl bromide gives ipratropium bromide. [2]
Therapeutic Uses
Ipratropium bromide is used for controlling and prevention of symptoms of:
- Wheezing
- Shortness of breath
- Chronic obstructive pulmonary disease like bronchitis and emphysema
Side Effects
Side effects of Ipratropium bromide are:
- Dry mouth
- Dizziness
- Stomach upset
- Constipation
- Drowsiness
- Allergic reactions
MCQ
Q.1 What can be the correct IUPAC nomenclature of Ipratropium bromide?
a) (RS)-1-(1-methylethylamino)-3-(1-naphthyloxy)propan-2-ol.
b) [8-methyl-8-(1-methylethyl)- 8-azoniabicyclo[3.2.1] oct-3-yl] 3-hydroxy-2-phenyl-propanoate
c) (RS)-(8-Methyl-8-azabicyclo[3.2.1]oct-3-yl) 3-hydroxy-2-phenylpropanoate
d) 2-[(Aminocarbonyl)oxy]-N,N,N-trimethylethanaminium chloride.
Q.2 Which amongst the following statements is/are incorrect related to the SAR of Ipratropium bromide?
I. X can also be either oxygen or absent completely.
II. The N substituent can be quarternary ammonium salt or tertiary amine or both with different alkyl groups.
III. Minimum potency obtained when the distance between the ring substituted carbons is 2 carbon units.
a) III
b) I
c) II, I
d) II, III
Q.3 The interaction of Atropine and isopropylbromide produces?
a) Ipratropium bromide
b) Carbachol
c) Procyclidine
d) Doxacurium
Q.4 Side effects of drug Ipratropium bromide is/are?
a) Allergic reactions
b) Constipation
c) Dryness in mouth
d) All of the above
Q.5 Match the following drugs with correct number of chiral carbons they are having.
| i. Propranolol | A. 1 |
| ii. Carbachol | B. 4 |
| iii. Ipratropium bromide | C. 0 |
| iv. Atropine | D. 5 |
a) i-A, ii-C, iii-B, iv-D
b) i-D, ii-,B iii-A, iv-C
c) i-B, ii-D, iii-A, iv-C
d) i-A, ii-C, iii-D, iv-B
Q.6 An example of drug from class Acetylcholie Antagonist (muscarinic antagonist) is?
a) Donepezil
b) Carbachol
c) Ipratropium
d) Paraoxin
Q.7 The type of ring system found in Ipratropium?
a) Benzene
b) Napthalene
c) Imidazole
d) None of the above
ANSWERS
1-b
2-a
3-a
4-d
5-d
6-c
7-a
REFERENCES
[1] Baigelman W, Chodosh S. Bronchodilator action of the anticholinergic drug, ipratropium bromide (Sch 1000), as an aerosol in chronic bronchitis and asthma. Chest. 1977 Mar 1;71(3):324-8. [2] Abdine HH, Belal F, Al-Badr AA. Ipratropium Bromide: Methods of Chemical and Biochemical Synthesis. Profiles of Drug Substances, Excipients and Related Methodology. 2003 Jan 1;30:85-99.