IVERMECTIN Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
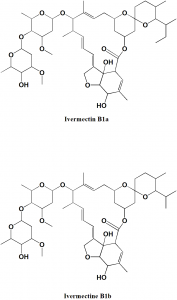
Ivermectin
IUPAC nomenclature
22,23-dihydroavermectin B1a + 22,23-dihydroavermectin B1b.
Classification
Ivermectin is an avermectin analogue. It is a broad spectrum anti-parasite medication. Antihelmintics.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 875.1g/mol |
| 2 | Physical appearance | White to yellowish white crystalline powder |
| 3 | Melting point | 155°C |
| 4 | Solubility | 0.005mg/ml in water |
| 6 | Presence of ring | Furane, pyrane |
| 7 | Number of chiral centers | 20 |
Mechanism of Action
- Ivermectin selectively binds with glutamate-gated chloride ion channels in invertebrate muscle and nerve cells of microfilaria and increases the permeability of the cell membrane to chloride which results in hyperpolarisation of the cell. This leads to the paralysis of the parasite and ultimately death.
- It also acts as agonist of the neurotransmitter GABA, disrupting GABA-mediated central nervous central neurosynpaptic transmission.
- It also disturbs he intrauterine development of microfilariae and inhibits their release from uteri of gravid female worms.
Structure Activity Relationship
- 4’-O-substituted derivatives retains biological activity.
- 5-O-substituted analogue has no biological activity.
- Presence of hydroxy or methoxy group at 5-position is essential for antiparasitic activity.
- The presence of two sugar moieties at position 13 is essential for biological activity.
- Reduction of the 22,23 position of avermectines shows better safety index
- Aliphatic nature of the ring having 5-OH/OMe is necessary for antiparasitic activity. If the ring is replaced by aromatic ring, then there is no activity. [1]
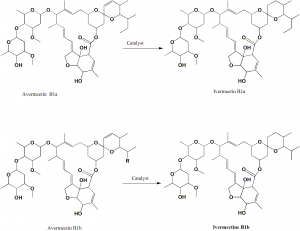
Method of synthesis
Selective, catalytic hydrogenation of the cis-22,23-double bond of the avermectins N1a and B1b. Catalyst used is Wilkinson’s catalyst chlorotris(triphenylphosphine)rhodium(I) {RhCl(PPh3)3].
Therapeutic Uses
Ivermectin is used for the treatment of certain parasitic roundworm infections.
Currently investigated for COVID-19 Treatment in Australia
Side Effects
Side effects of Ivermectin are:
- Nausea
- Muscle pain
- Diarrhea
- Dizziness
- Headache
- Joint pain
- Swelling in lymph nodes
- Eyes swelling
- Weakness
- Vision changes
- Itching
- Rashes
- Fever
MCQ
Q.1 What can be the correct IUPAC nomenclature of the drug ivermectn?
a) 22,23-dihydroavermectin B1a
b) 22,23-dihydroavermectin B1b
c) Both a) and b)
d) None of the above
Q.2 Which amongst the following statements is/are incorrect related to the SAR of ivermectin?
I. 4’-O-substituted derivatives retains biological activity.
II. 5-O-substituted analogue has maximum biological activity.
III. Presence of hydroxy or methoxy group at 5-position is essential for antiparasitic activity.
IV. The presence of two sugar moieties at position 13 is essential for biological activity.
a) I
b) II
c) III
d) IV
Q.3 Ivermectin can be synthesized from avermectin by ?
a) Hydrogenation
b) Oxidation
c) Chlorination
d) Alkylation
Q.4 Side effects of drug Ivermectin is/are?
a) Headache
b) Swelling of lymph nodes
c) Diarrhea
d) All of the above
Q.5 Match the following drugs with their correct molecular weights-
| i. Ivermectin | A. 875.1 gm/mol |
| ii. Chloraquine | B. 319.9 gm/mol |
| iii. Nitrazepam | C.281.27 gm/mol |
| iv. Zaleplon | D. 305.33 gm/mol |
a) i-C, ii-B, iii-D, iv-A
b) i-A, ii-B, iii-C, iv-D
c) i-B, ii-D, iii-C, iv-A
d) i-D, ii-A, iii-C, iv-B
Q.6 An example of drug from class antihelminthics ?
a) Ivermectin
b) Diazepam
c) Fludarabine
d) Pentobarbital
Q.7 Number of chiral centers present in the structure of Ivermectin is?
a) 5
b) 10
c) 15
d) 20
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-c
2-b
3-a
4-d
5-b
6-a
7-d