LABETOLOL Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
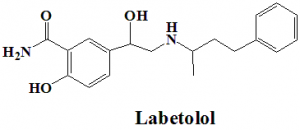
Labetolol
IUPAC nomenclature
(RS)-2-Hydroxy-5-[1-hydroxy-2-[(4-phenylbutan-2-yl)amino]ethyl]benzamide.
Classification
Labetolol is a mixed α/ß blocker.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 328.4 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | 188°C |
| 4 | Solubility | Water solubility is 3.56e-04 M |
| 5 | Octanol/water partition coefficient | 2.7 |
| 6 | Presence of ring | Benzene |
| 7 | Number of chiral centers | 2 |
Mechanism of Action
- Labetolol selectively antagonizes α1– adrenergic receptors and it nonselectively antagonizes ß-adrenergic receptors.
- It has having 3 times ß-blocking activity than α-blocking activity.
- Antagonism of α-receptors leads to vasodilation and decreased vascular resistance, which further results in decreased blood pressure.
- Antagonism of ß1-adrenergic receptors produces slight decrease in heart rate.
- Antagonism of ß2-adrenergic receptors is responsible for the side effects of the drug like bronchospasms.
- Labetolol produces a sustained vasodilation for a longer duration without the significant lowering in cardiac output or stroke volume, and a slight decrease in heart rate.
Structure Activity Relationship
- The type of N-substitutions such as N-isopropyl and N-t-butyl eliminates α1-activity.
- Arylalkyl groups with α-methyl substituent returns back the α1-affinity but not the intrinsic activity.
- The ß-blocking activity is almost 1.5 folds that of its α1-blocking activity.
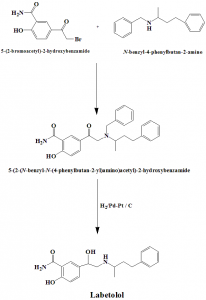
Method of synthesis
i. N-alkylation of N-benzyl-N(4-phenyl-2-butyl)amine 5-bromoacetylsalicylamide forms aminoketone.
ii. Debenzylation of aminoketone by hydrogen using palladium-platinium on carbon catalyst yields labetolol.
Therapeutic Uses
Labetolol is used for treatment of:
- Hypertension
- Prevention of strokes
- Prevention of heart attacks
- Prevention of kidney problems
Side Effects
Side effects of Labetolol are:
- Dizziness
- Tiredness
- Scalp tingling
- Decreased libido
- Lightheadedness
MCQs
Q.1 Choose the correct option related to the correct physicochemical properties of drug labetolol.
I. Molecular weight is 266.34 gm/mol.
II. It is present in solid form.
III. Melting point is 188°C
a) I, II, III
b) II, III
c) I, III
d) I, II
Q.2 Match the following of the drugs with their correct IUPAC names.
| i. Atenolol | A. (±)-[3-(9H-carbazol-4-yloxy)-2-hydroxypropyl][2-(2-methoxyphenoxy)ethyl]amine |
| ii. Labetolol | B. (RS)-2-Hydroxy-5-[1-hydroxy-2-[(4-phenylbutan-2-yl)amino]ethyl]benzamide |
| iii. Bisoprolol | C. (RS)-1-{4-[(2-Isopropoxyethoxy)methyl]phenoxy}-
3-(isopropylamino)propan-2-ol |
| iv. Carvedilol | D. (RS)-2-{4-[2-Hydroxy-3-(propan-2-ylamino)propoxy]phenyl}acetamide. |
a) i-B, ii-A, iii-C, iv-D
b) i-D, ii-A, iii-B, iv-C
c) i-A, ii-C, iii-D, iv-B
d) i-D, ii-A, iii-C, iv-B
Q.3 Pick the correct statement related with the mechanism of action of drug labetolol?
a) It selectively antagonizes α1-adrenergic receptors and nonselectively antagonizes ß-adrenergic receptors .
b) It nonselectively antagonizes α-adrenergic receptors and nonselectively antagonizes ß-adrenergic receptors .
c) It selectively antagonizes α2-adrenergic receptors and selectively antagonizes ß2-adrenergic receptors .
d) It nonselectively antagonizes α-adrenergic receptors and nonselectively antagonizes ß-adrenergic receptors .
Q.4 Correct sequence for True/false for the classification of the drug can be?
- Atenolol: selective ß1-adrenergic antagonist.
- Labetolol: mixed α/ß blocker
- Salmeterol: nonselective adrenergic agonist.
- Methyldopa: nonselective α-adrenergic antagonist
a) TTFF
b) TTFT
c) TFTF
d) FFTT
Q.5 Complete the following sentence related with structure activity relationship of Labetolol-
‘The ß-blocking activity is almost ………. That of α-blocking activity.
a) Half
b) Quarter
c) Double
d) 1.5 folds
Q.6 The correct sequence for the steps for synthesis of drug Labetolol from N-benzyl-N(4-phenyl-2-butyl)amine 5-bromoacetylsalicylamide
I. N-alkylation
II. Debenzylation
III. Hydrolysis
a) I – II
b) III – II
c) II – I
d) II – III
Q.7 Side effect of drug Labetolol?
a) Lightheadedness
b) scalp tingling
c) Decreased libido
d) All of the above
FREE GPAT online Test: Participate: Click Here
ANSWERS
1-b
2-d
3-a
4-a
5-c
6-a
7-d
REFERENCES
[1] Carey B, Whalley ET. β‐Adrenoceptor agonist activity of labetolol on the isolated uterus of the rat. British journal of pharmacology. 1979 Sep;67(1):13-5. [2] Lemke TL, Williams DA, Foye WO. Principles of medicinal chemistry. Williams & Wilkins; 2017, 356. [3] Vardanyan R, Hruby V. Synthesis of essential drugs. Elsevier; 2006 Mar 10.