LETROZOLE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
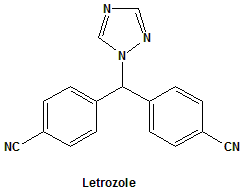
Letrozole
IUPAC nomenclature
4,4′-((1H-1,2,4-triazol-1-yl)methylene)dibenzonitrile.
Classification
Letrozole is an aromatase inhibitor. [1]
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 285.3 g/mol |
| 2 | Appearance | White to yellow crystalline powder.
It is present in anhydrous crystal form |
| 3 | Melting point | 185 °C |
| 4 | Solubility | Practically insoluble in water |
| 5 | Octanol/water partition coefficient | 2.5 |
| 6 | Presence of ring | Bezonitrile and triazole rings |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
i. Letrozole blocks the active site of CYP19A1.
ii. Electron transfer chain is also blocked.
iii. This leads to the competitive inhibition, and thus, prevention of conversion of androgens to estrogen.
iv. This results in elevation of leuteinizing hormone and reduction in uterine weight.
v. Due to reduced availability of estrogen, estrogen –dependent tumors regress.
Structural Activity Relationship
- At least a carbonyl group at C-3 or C-17 is required. It will be better if both are present. If one of the carbonyl group is required to be loosed then, it will be preferred to keep the C-17 carbonyl group.
- Enough planarity should be given in A-ring and A,B-ring junction by providing double bonds or other functions. Planarity is important for the inhibition of aromatase.
- Extend and conjugate the number of double bonds in convenient positions of A and B rings of the steroid at positions C-1, C-4 and C-6 can increase the potency of the drug.
- Bulky groups decreases the activity or binding of the drug.
- Substitutions at C-6 and C-7 positions can increase the aromatase inhibition property. [2]
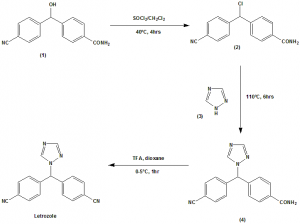
Method of synthesis
i. The alcohol compound (1) treated with thionyl chloride in dichloromethane to give compound (2) in excellent yield.
ii. (2) is treated with 1H-1,2,4-triazole (3) at higher temperature in presence of polar protic solvents to give 4-(4-cyanobenzoyl) benzamide (4).
iii. The amide of (4) will be converted to nitrile using triflouroacetic anhydride in 1,4-dioxane to give letrozole. [3]
Therapeutic Uses
The drug used for the treatment of:
- Breast cancer in postmenopausal women.
Side Effects
- Common side effects of letrozole include hot flashes and higher level of cholesterol.
- Less common side effects are nausea, weight gain, night sweats and arthralgias.
MCQs
Q.1 “4,4′-((1H-1,2,4-triazol-1-yl)methylene)dibenzonitrile” is the IUPAC nomenclature of which drug?
a) Letrozole
b) Dutasteride
c) Busulfan
d) Paclitaxel
Q.2 Predict the correct statement related to the therapeutic uses of drug Letrozole-
a) Letrozole is used as an antibiotic.
b) Letrozole is used for treatment of breast cancer.
c) Letrozole is most common drug used for the treatment of Rhabdomyosarcoma.
d) Both a) and b)
Q.3 Match the following with respect to the SAR of drug Letrozole-
| i. At least a carbonyl group is required at ……… or ………. for the activity of drug | A. C-3 , C-17 |
| ii. Carbonyl group at ……… is preferred to keep for the better activity. | B. C-6, C-7 |
| iii. Substitution at ……… and ……. Can increase the aromatase inhibition property. | C. C-7 |
| D. C-17 |
a) i-A, ii-D, iii-B
b) i-B, ii-C, iii-A
c) i-A, ii-C, iii-B
d) i-B, ii-D, iii-A
Q.4 Which amongst the following drugs shows its effect through inhibition of aromatase enzyme?
a) Carmustine
b) Methotrexate
c) Letrozole
d) Finasteride
Q.5 Letrozole drug belongs to which class?
a) Progestins
b) GnRH analogues
c) Antiandrogens
d) Aromatase inhibitors
Q.6 Which of the following is not a side effect of Letrozole?
a) Low cholesterol
b) Hot flashes
c) Weight gain
d) Night sweats
Q.7 How many rings are present in the structure of letrozole?
a) 0
b) 1
c) 2
d) 3
ANSWERS
1-a
2-b
3-a
4-c
5-d
6-a
7-d
REFERENCES
[1] Tripathi KD. Essentials of Medical Pharmacology, 6thEdn. Jaypee Brothers Medical Publishers (P) Ltd. 2008: 820. [2] Varela C. Design, synthesis and structure-activity relationships studies on steroidal aromatase and 5α-reductase inhibitors as anti-tumors (Doctoral dissertation). [3] Suman M, Vijayabhaskar B, NageswaraRao K, Kumar US, VenkateswaraRaoe B. A novel process for the synthesis of substantially pure Letrozole. Organic Chemistry. 2019 Jan 1(part v):319-26.